Sir Richard Dearlove, 75, a four decade veteran of the intelligence services said in the Telegraph’s new Planet Normal podcast, which you can listen to on the audio player above, that a scientific paper published this week by a Norwegian-British research team claims to have discovered clues within SARS-CoV-2's genetic sequence about its origin. He suggested this new information showed key elements of the virus had been "inserted" and may not have evolved naturally. The coronavirus pandemic "started as an accident" when the virus escaped from the laboratory in China, he said. Sir Richard described the controversial study as "a very important contribution to a debate which is now starting about how the virus evolved and how it got out and broke out as a pandemic," adding: "I think this particular article is very important, and I think it will shift the debate." Sir Richard was the head of MI6 between 1999 and 2004. Listen to the Planet Normal interview with Sir Richard Dearlove on the audio player above, or susbscribe to the podcast here.
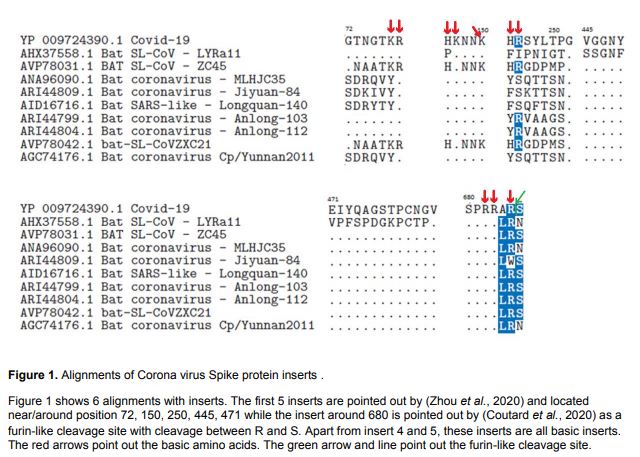
THE INSERTION
Furthermore the insertion of a multibasic motif RERRRKKR?GL at the H5N1 hemagglutinin HA cleavage site was likely associated with the hyper-virulence of the virus during the Hong Kong 1997 outbreak. Extensive clinical evidence in this pandemic suggests that SARS-CoV-2 poses such widened cell tropism. The co-receptor dependent phagocytic general method of action of SARS-CoV-2 appears to be specifically related to cumulative charge: please refer to SARS-CoV-2 peaks above pI=8.24 compared to human SARS-CoV. These basic domains - partly inserted and partly substituted amino acids - explain the salt bridges formed between the SARS-CoV-2 Spike and its co-receptors on the cell membrane. Indeed, these data suggest that the infectivity of SARS-CoV-2 is best explained by this cumulative charge associated with these basic charged domains, enabling extra salt bridges to attach to membrane components as well as to the membrane itself.
We have earlier explained the enhanced presence of basic amino acids in the inserts such as Lysine (K) and Arginine (R) and their association with enhanced pathogenicity in other pathogens like the 1997 H7N1 Hong Kong Flu. We noted above the critical importance of understanding that cumulative positive charge associated with the inserted short sections has the effect of enabling extra salt bridges to attach to the membrane. Under this general method of action, this combination of basic amino acids in the SARS-CoV-2 Spike binds to cells in the upper airways. Its high infectivity is associated with olfaction and taste; and systemic release of the virus explains the clinical findings associated with destruction of erythrocytes T-cells and cells associated with neuropathological conditions. See original peer-reviewed Cambridge University paper.
MISC.
ANOTHER POSSIBILITY WHICH STILL CANNOT BE EXCLUDED IS THAT SARS-CoV-2 WAS CREATED BY A RECOMBINATION EVENT THAT OCCURRED INADVERTENTLY OR CONSCIOUSLY IN A LABORATORY HANDLING CORONAVIRUSES, WITH THE NEW VIRUS THEN ACCIDENTALLY RELEASED INTO THE LOCAL HUMAN POPULATION."
DO-IT-YOURSELF DNA RECOMBINANT KIT: CREATE A NEW VIRUS

PROFESSOR SHI ZHENG LI IS THE NUMBER ONE SUSPECT IN THE CREATION OF SARS-CoV-2, STILL NOT FOUND IN AN INTERMEDIATE ANIMAL THE VIRUS WAS CREATED AT THE WUHAN INSTITUTE OF VIROLOGY (WIV)
IN A BIO-SAFETY LEVEL TWO LABORATORY (BSL-2) RATHER THAN BSL-4 LAB WHICH IS USED FOR EXPERIMENTS WITH VIRUSES THAT CAUSE INCURABLE DISEASES MAKING IT EASIER FOR THE VIRUS TO ESCAPE.THE FRENCH HELP WIV BUILD THE LEVEL 4 BIOCONTAINMENT LAB DESPITE DUAL USE CONCERNS

In 2004, the Chinese and French governments signed a cooperation agreement on fighting and preventing new diseases, stressing the active cooperation between China and France in the construction of high-level biosafety laboratories and the system construction of biosafety laws and regulations etc. In order to implement the spirit of Sino-French agreement, in 2005, Wuhan Institute of Virology undertook the task of building a national biosafety laboratory of Wuhan, Chinese Academy of Sciences. With nearly 10 years of unremitting efforts, the laboratory completed the physical facilities in January 2015. In August 2016, it obtained the recognition and authentication certificate for the critical protection equipment installation and commissioning.

DEFENSE, EXTERNAL INTELLIGENCE (DGSE) AND FOREIGN AFFAIRS WERE STANDING AGAINST A PROJECT THAT COULD SERVE A MILITARY BACTERIOLOGICAL WEAPON PROGRAM.
At the time when it was launched by Jacques Chirac and his Prime Minister Jean-Pierre Raffarin, in 2004, the P4 was at the heart of a showdown in France. Those in favor, politicians and scientists, said that China, which had barely emerged from SARS (Severe Acute Respiratory Syndrome), should be helped to defend itself against epidemics. But Defense, External Intelligence (DGSE) and Foreign Affairs were standing against a project that they believed could serve a military bacteriological weapon program. They suspected that Beijing would want to equip itself with five or seven P4 laboratories, including two for military purposes. "We knew the risks involved and thought that the Chinese would control everything and quickly eject us from the project. We believed that providing this cutting-edge technology to a country with an infinite power agenda risked exposing France in return, ”said a diplomat who followed the case closely. According to a high-ranking source, the project would also have provoked a crisis in Pasteur, where the Assembly of the Hundred, the parliament of the Institute, would have denounced the access authorized by the contract to some of its databases, before see the decision imposed by its management. " There were arguments, because China has a real medical problem with epidemics and France had an advantage in this technology very advanced. But the Chinese know how to copy and duplicate. And we thought the P4 would give China instruments if it ever wanted to start a biological weapons program, "said a senior diplomat, who was in strategic affairs at the time. French scholars played a big role in pushing the project. " There was a blindness of the scientific community which refused to see the reality of the Chinese system. Researchers believed that openness to capitalism would transform China into a normal country. It was to forget that it remained above all a Leninist state in which science is not independent but directed by the Communist Party ", explains Valérie Niquet, specialist in Asia at the Foundation for Strategic Research (FRS). Since the start of the epidemic, the party and the state have been involved in research, manipulating dates and rewriting the history of the coronavirus.
CHINESE RESPONSE
On April 21, Le Figaro published an article "Investigation: How the P4 laboratory in Wuhan, exported by France, escaped all control" signed by Isabelle Lasserre. Some comments in the article are shocking. The Chinese Embassy in France wants to question them. She wrote in the article that: "On February 16, Chinese state media also reported deficiencies. They claimed in particular that researchers threw laboratory materials down the drain, after experimentation and without having subjected them to the specific treatment intended for biological discharges. They also pointed out that a certain number of researchers, to make ends meet, were selling laboratory animals which had undergone experiments on the markets of Wuhan. As anyone with common sense knows very well, a high-level laboratory is endowed with rigorous management systems and codes of conduct for scientific research, how is it possible that the basic faults described in the article appear? This is an extremely serious problem which seriously damages the reputation of Chinese research institutions and the national image of China. We ask the journalist to tell her readers specifically that her comments come from which media and which reports. Otherwise, they can only be considered as lies. The author of the text made many criticisms of the joint construction of the P4 laboratory in Wuhan by China and France. It is his personal opinion, in which the Embassy does not intervene. But the descriptions in the article are very likely to misinform readers, and do not promote mutual knowledge of the Chinese and French peoples and friendly cooperation between the two countries. The Chinese Embassy in France recommends the video of an interview given by Mr. Yuan Zhiming, Director of the Wuhan branch of the Chinese Academy of Sciences and researcher at the Wuhan Institute of Virology, on April 20 at CGTN. The author of the article could undoubtedly obtain useful information in this, which would help him to know the question concerned in a more complete and more precise way.

Yuan Zhiming: The core of the Wuhan BSL-4 Laboratory is surrounded by stainless-steel walls, forming a "box-within-a-box" structure. The core lab enclosure can ensure sufficient structural strength and tightness to form a static seal. The lab's dynamic seal uses negative pressure technology to ensure a strict and orderly pressure gradient between the functional areas, thereby effectively preventing any air contaminated by infectious pathogenic microorganisms from spreading to areas with low contamination probability and to the external environment.
Air emitted from the lab is filtered and discharged by two-stage high-efficiency filters to ensure the safety of the emissions. Waste water is discharged after high-temperature treatment in a sewage treatment system. Polluted waste in the lab is subjected to high-temperature and high-pressure treatment by double-door autoclaves, and then safely removed and delivered to a centralized medical waste disposal unit with corresponding qualifications for disposal. Whenever personnel pass through the entrance and exit channels, their positive pressure protective suits are chemically disinfected using the chemical showers to ensure the safety of the passageways. The above technical protection measures ensure that viruses inside the lab cannot escape.
Plum Island Reports Disease Outbreak By John Rather Aug. 22, 2004 THE Department of Homeland Security confirmed last week that the highly contagious foot-and-mouth virus had briefly spread within the Plum Island Animal Disease Center in two previously undisclosed incidents earlier this summer. A spokesman for the department, which took over the high-security laboratory from the Department of Agriculture in June 2003, said safety procedures had been stepped up and laboratory rooms disinfected after the incidents, which occurred on June 24 and July 19.
Documentary evidence indicates that the novel-bat-virus projects at Wuhan CDC and the Wuhan Institute of Virology used personal protective equipment and biosafety standards that would pose high risk of accidental infection of a lab worker upon contact with a virus having the transmission properties of the outbreak virus. Official Chinese government recognition early in the SARS-CoV-2 outbreak of biosafety inadequacies in China’s high containment facilities. In February 2020, several weeks after the outbreak of the disease in Wuhan, China’s President Xi Jinping stressed the need to ensure “biosafety and biosecurity of the country.” This was followed immediately by a China Ministry of Science & Technology announcement of new guidelines for laboratories, especially in handling viruses. Almost at the same time, the Chinese newspaper Global Times published an article on “chronic inadequate management issues at laboratories, including problems of biological wastes.” Documentary evidence indicates that the novel-bat-virus projects at Wuhan CDC and the Wuhan Institute of Virology used personal protective equipment and biosafety standards that would pose high risk of accidental infection of a lab worker upon contact with a virus having the transmission properties of the outbreak virus. BULLETIN OF ATOMIC SCIENTISTS Relying on the major science and technology infrastructure, this project aims to cultivate national high-level biosafety talents, to output significant scientific and technological breakthroughs and achievements, and to promote the scientific and technological support capabilities for biosafety and public health. According to the scientific and technological development programs of China, Chinese Academy of Sciences (CAS) and Wuhan Institute of Virology (WIV), CAS, the Call Announcement 2020 of Advanced Customer Cultivation Project of Wuhan National Biosafety Laboratory, CAS is released. Please apply for the project accordingly. The specific contents are as below...The research achievements attained during the project implementation, including theses, monographs, patents, software and database etc. shall be marked with “Funded by Advanced Customer Cultivation Project of Wuhan National Biosafety Laboratory, Chinese Academy of Sciences”. Any achievements unmarked will not be counted in the assessment. Wuhan Institute of VirologyChinese Academy of Sciences Jan 15th, 2020 One component of the novel-bat-virus project at the Wuhan Institute of Virology involved infection of laboratory animals with bat viruses. Therefore, the possibility of a lab accident includes scenarios with direct transmission of a bat virus to a lab worker, scenarios with transmission of a bat virus to a laboratory animal and then to a lab worker, and scenarios involving improper disposal of laboratory animals or laboratory waste. BULLETIN OF ATOMIC SCIENTISTS
SHI ZHENG LI or Shi Zhengli or Dr. Zheng-li Shi (AKA "The Bat Lady") Director of the Centre for Emergence of Infectious Disease and Biosafety at the Wuhan Institute of Virology, a Biosafety Level Four Biocontainment Lab, where diseases with no known cures are studied, is the number one suspect in the creation of the SARS-CoV-2 virus. According to her the pandemic is "nature's punishment for an unsanitary lifestyle." She had the skills, 24 hour access to https://bit.ly/2QeJEW7the equipment needed to create the virus, and could have easily done it on her own. As Director of Biosafety SHI ZHENG LI
conducted her experiments in the Level 2 Bio-containment part of WIV rather than the higher security Bio Safety Level 4 lab despite the fact the the viruses could have caused a pandemic. The WIV was established in order to study the SARS viruses after the first SARS outbreak in 2003. However, it became a SARS virus factory. SARS viruses were the subject of Gain-of-Function (GoF) experiments in order to make them "gain an additional function" and become more lethal and contagious thus creating NOVEL or new viruses. This was a highly controversial proceedure that set off alarms among scientists. SO - there just happened to be a SARS virus factory in the same city where an outbreak of SARS-CoV-2, a Novel virus, took place. How could this be a coincidence? Nature Magazine thinks so: "That the WIV, a laboratory highly regarded for its work on bat coronaviruses, is located in the city where the outbreak first emerged is probably just a coincidence."
WIV stressed that their aim of research on coronaviruses was to find vaccines and antivirus drugs to deal with possible future outbreak due to a new attack of coronaviruses on humans. The HKC News reported:
"In the last 14 years, WIV has published a rich pool of papers on the study of coronavirus and made a collection of viruses from bats in their habitat. However, we have so far not witnessed the department under SHI ZHENG LI, publish any research paper on vaccines and antiviral drugs countering the coronaviruses they have collected from nature and laboratory synthesized. SHI ZHENG LI and her teammate's warned of the potential outbreak of disease caused by a new coronavirus with high infectivity and pathogenicity were not acted upon and vaccines and drugs they promised were not being delivered." In 2004, according to Scientific American, a team from the WIV led by virologist SHI ZHENG LI began visiting caves in southern China, hoping to find the cause of SARS. They captured bats and took blood, saliva, and fecal samples, and tested the samples for viruses back in Wuhan. Shi became known as the Chinese Bat Lady. In 2009, the lab began working with PREDICT, a new program established at USAID to train and fund scientists to test “high-risk” areas for new viruses. By identifying unknown viruses before they spilled over into humans—to “find them before they find us,” as SHI ZHENG LI put it—researchers hoped to establish an early-warning system. PREDICT worked in dozens of countries, but the WIV was one of its linchpins, and SHI ZHENG LI became famous as China’s “Bat Woman.”
In 2013, the WIV discovered SARS-CoV, the cause of SARS, in a cave in Yunnan Province. SHI ZHENG LI team found that the bats of southern China were full of viruses, especially coronaviruses. Over 10 years, her team collected more than 10,000 samples from bats in the region and discovered hundreds of new corona viruses, including some with the ability to infect humans. Many bats harbored multiple viruses, and there were alarming signs that the viruses were recombining with each other—swapping chunks of genetic code as they replicated, producing novel viruses with new abilities. [However it would take 50 years for SARS-CoV-1 to mutate into SARS-CoV-2 and there was no guarantee this would happen.]
SHI ZHENG LI During the past two decades, three zoonotic coronaviruses have been identified as the cause of large-scale disease outbreaks–Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and Swine Acute Diarrhea Syndrome (SADS). SARS and MERS emerged in 2003 and 2012, respectively, and caused a worldwide pandemic that claimed thousands of human lives, while SADS struck the swine industry in 2017. They have common characteristics, such as they are all highly pathogenic to humans or livestock, their agents originated from bats, and two of them originated in China. Thus, it is highly likely that future SARS- or MERS-like coronavirus outbreaks will originate from bats, and there is an increased probability that this will occur in China. Therefore, the investigation of bat coronaviruses becomes an urgent issue for the detection of early warning signs, which in turn minimizes the impact of such future outbreaks in China. The purpose of the review is to summarize the current knowledge on viral diversity, reservoir hosts, and the geographical distributions of bat coronaviruses in China, and eventually we aim to predict virus hotspots and their cross-species transmission potential. In 2012, Shi's colleague has extracted 27 isolates of SARS-like CoVs from 117 samples of bats anal swab and faeces that they collected from a Yunnan bat cave. They divided them into seven groups. Each group of strains belongs to one species. Of these seven groups, one type of strains can directly infect human without going through any animal host, which they registered in a Gene Bank as WIV1 ( Wuhan Institute of Virology #1); and two types have a similarity of 95% to SARS-CoV, as compared with most other SARS-like CoVs which have similarities in the range of 76-92% in full genome comparison. They registered them as Rs3367 and SHC014. Later they used the SHC014 to create an artificial coronavirus (SHC014-MA15) which has high pathogenicity on humans.
October 30, 2013 SHI ZHENG LI and PETER DASZAK team up again to author a Nature article, entitled Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor — offering strongest evidence to date that Chinese horseshoe bats are natural reservoirs of SARS-CoV.
February 22, 2017 The Wuhan Institute of Virology, China’s first biosafety level-4 lab (BSL-4), is certified to work on the most dangerous pathogens. Nature, “But worries surround the Chinese lab, too. The SARS virus has escaped from high-level containment facilities in Beijing multiple times.” The Wuhan Institute of Virology is the only BSL-4 virology lab in China staffed with two Chinese virologists, SHI ZHENG LI and XING-YI GE who both previously worked at a University of North Carolina at Chapel Hill lab, which had already bio-engineered an incredibly virulent strain of bat coronavirus.
November 30, 2017 Nature: “After a detective hunt across China, researchers chasing the origin of the deadly SARS virus have finally found their smoking gun. In a remote cave in Yunnan province, virologists have identified a single population of horseshoe bats that harbours virus strains with all the genetic building blocks of the one that jumped to humans in 2002, killing almost 800 people around the world.” That report was based on an article, entitled, “Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus,” published in San Francisco-based PLOS Pathogens and jointly funded by National Natural Science Foundation of China. And wielding the smoking gun, besides a cast of usual suspects (Xing-Yi Ge and PETER DASZAK ) was none other than SHI ZHENG LI, a virologist who is often called China’s “bat woman” by her colleagues because of her virus-hunting expeditions in bat caves over the past 16 years."
January 19, 2018 U.S. Embassy in Beijing takes unusual step of repeatedly sending U.S. science diplomats to the Wuhan Institute of Virology. One visit raises so many red flags, that a cable is sent noting, “a serious shortage of appropriately trained technicians and investigators needed to safely operate this high-containment laboratory.” The U.S. Counselor visited Wuhan Institute of Virology, CAS (March 3, 2018). On March 27th, Mr. Rick Switzer, Counselor of Environment, Science, Technology and Health Section of Embassy of the United States in China, accompanied by the U.S. Consul General in Wuhan, Mr. Jamison Fouss, visited Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS). Prof. YANYI WANG, the Deputy Director General of the WIV, met with the U.S. delegation. Prof. SHI ZHENG LI, Director of Center for Emerging Infectious Diseases and Deputy Director of Wuhan P4 Laboratory, and Prof. Zhihong Hu, Director of Center for Bacteria and Virus Resources and Application, participated the meeting. On March 27th, Mr. Rick Switzer, Counselor of Environment, Science, Technology and Health Section of Embassy of the United States in China, accompanied by the U.S. Consul General in Wuhan, Mr. Jamison Fouss, visited Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS). Prof. YANYI WANG, the Deputy Director General of the WIV, met with the U.S. delegation. Prof. ZHENGLI SHI, Director of Center for Emerging Infectious Diseases and Deputy Director of Wuhan P4 Laboratory, and Prof. Zhihong HU, Director of Center for Bacteria and Virus Resources and Application, participated the meeting. Source: The U.S. Counselor visited Wuhan Institute of Virology, CAS (March 3, 2018)
March 27, 2018 The Wuhan Institute of Virology issues a news release in English after the last of the U.S. Embassy visits. The news release was removed the week of April 6, 2020, although it remains archived on the internet.
2019 SHI ZHENG LI group was creating chimeric constructs as far back as 2007 and as recently as 2017, when they created a whole of 8 new chimeric coronaviruses with various RECEPTOR BINDING MOTIFS. In 2019 such work was in full swing, as WIV was part of a $3.7 million NIH grant titled Understanding the Risk of Bat Coronavirus Emergence. Under its auspices, SHI ZHENG LI co-authored a 2019 paper that called for continued research into synthetic viruses and testing them in vitro and in vivo:
"Currently, no clinical treatments or prevention strategies are available for any human coronavirus. Given the conserved receptor-binding domains of SARS-CoV and bat SARSr-CoVs, some anti-SARS-CoV strategies in development, such as anti-RECEPTOR BINDING DOMAIN antibodies or RECEPTOR BINDING DOMAIN-based vaccines, should be tested against bat SARSr-CoVs. Recent studies demonstrated that anti-SARS-CoV strategies worked against only WIV1 and not SHC014. In addition, little information is available on HKU3-related strains that have much wider geographical distribution and bear truncations in their receptor-binding domain. Similarly, anti-S antibodies against MERS-CoV could not protect from infection with a pseudovirus bearing the bat MERSr-CoV S. Furthermore, little is known about the replication and pathogenesis of these bat viruses. Thus, future work should be focused on the biological properties of these viruses using virus isolation, reverse genetics and in vitro and in vivo infection assays. The resulting data would help the prevention and control of emerging SARS-like or MERS-like diseases in the future." If the above quote might seem vague as to what exactly “using reverse genetics” might mean, the NIH grant itself spells it out: Aim 3. In vitro and in vivo characterization of SARSr-CoV spillover risk, coupled with spatial and phylogenetic analyses to identify the regions and viruses of public health concern. We will use S protein sequence data, infectious clone technology, in vitro and in vivo infection experiments and analysis of receptor binding to test the hypothesis that % divergence thresholds in S protein sequences predict spillover potential. “Infectious clone technology” stands for creating live synthetic viral clones. Considering the heights of user friendliness and automation that genetic engineering tools have attained, creating a synthetic CoV2 via the above methodology would be in reach of even a grad student. But before delving into CoV2 origins, let’s first take a quick dive into its biology.
“It is highly likely that future SARS- or MERS-like coronavirus outbreaks will originate from bats, and there is an increased probability that this will occur in China,” SHI ZHENG LI and her colleagues wrote in a 2019 paper that now seems chillingly prescient. “Therefore, the investigation of bat coronaviruses becomes an urgent issue for the detection of early warning signs.”
1. You have published many papers on coronaviruses and even warned of the possibility of a serious spillover event from animals to humans. Do you feel like your warnings were not appropriately heeded and how does what imagined compare to the scale of this pandemic?
A: The risk of cross-species infection always exists. With regards to this issue, China has undertaken scientific research projects, created facilities and equipment, and built teams of experts. With global environmental change and the expansion of human activity, the risk of infection continues to increase, which is confirmed and supported by our research. However, our study did not involve an investigation into the pandemic’s scale.
["With global environmental change and the expansion of human activity..." Humans are building developments over bat caves? "However, our study did not involve an investigation into the pandemic’s scale." Unimportant to SHI ZHENG LI.]
2. How has the pandemic affected you, personally and professionally?
A: This pandemic has made me realize the importance of our work and the necessity of pursuing it. I think if we could do more basic research and technological development on vaccines and therapeutic drugs, we would do better in this regard.
[WIV never produced a vaccine or therapeutic - just Novel Viruses]
3. What are your leading theories about the origin of SARS-CoV-2 and has your own research shed any light on the question?
A: According to the findings of our team and our international peers, SARS-CoV-2 is very likely to have originated from bats. It may have evolved in one or more intermediate hosts, become adapted to humans, and eventually spread among humans. However, it remains unclear which animals were the intermediate hosts and how it spilled over to humans.
4. Assuming this was natural event, a virus jumping species somewhere, how do you think this likely happened? Do you think it’s possible that a bat in or close to Wuhan infected someone? If not, where could this have taken place? What do you think of the theory that infected people who lived near mines were the index cases and that they traveled to Wuhan?
A: There are two possibilities for the cross-species transmission from the natural host to humans. One is that the virus was transmitted directly from a bat to a human, while the other is that the virus spread to humans via one or more intermediate hosts. For SARS-CoV-2, though the first possibility cannot be ruled out, its likelihood is very low. I tend to support the second scenario. When and where the earliest cross-species transmission of SARS-CoV-2 occurred from the intermediate host to humans has not been scientifically uncovered yet. We know from historical experience like HIV that the places where big emerging diseases first break out usually are not their place of origin (where the spillover originally happened). Tracing the origin of a virus is a very challenging scientific task. As for the origin and transmission routes of SARS-CoV-2, it needs a pioneering vision, and the collective efforts of scientists all around the world, and it needs time as well. We have done bat virus surveillance in Hubei Province for many years, but have not found that bats in Wuhan or even the wider Hubei Province carry any coronaviruses that are closely related to SARS-CoV-2. I don't think the spillover from bats to humans occurred in Wuhan or in Hubei Province. I guess you are referring to the bat cave in Tongguan town in Mojiang county of Yunnan Province. To date, none of nearby residents is infected with coronaviruses. Thus the claim that the so-called "patient zero" was living near the mining area and then went to Wuhan is false.
5. An early cluster at the Huanan seafood market in Wuhan led many to think that an animal there somehow infected humans. How has your thinking about the seafood market’s role evolved as it became clear that many of the earliest cases are not linked to it?
A: As you pointed out, some early patients do not have a history of Huanan seafood market exposure. We detected SARS-CoV-2 nucleic acids in environmental samples from sources such as rolling door handles, the ground and sewage in that market, but we did not detect any SARS-CoV-2 nucleic acids in frozen animal samples. The Huanan seafood market may just be a crowded location where a cluster of early novel coronavirus patients were found.
6. Do you know whether anyone tested animals from the market? If not, why not?
A: Under the deployment of Hubei Provincial Government, our team, alongside researchers from Huazhong Agricultural University, collected environmental samples and frozen animal samples in Huanan seafood market. We detected SARS-CoV-2 nucleic acids only in the environmental samples such as roller shutter door handles, the ground and sewage, but not in the animals.
7. Is there an attempt to use registries from the market to test farms that supplied animals to the market? Has your group or any other done testing of domesticated animals or wild animal farms at any farms for SARS-CoV-2 like viruses? If so, what has research found?
A: Under the deployment of the Hubei Provincial [Communist authoritarian] Government, our team and researchers from Huazhong Agricultural University collected samples of farmed animals and livestock from farms around Wuhan and in other places in Hubei Province. We did not detect any SARSCoV-2 nucleic acids in these samples.
8. Were you ever given environmental or animal samples from the market to test yourself? If so, what did you find? If not, what do you know about the market samples tested?
A: We detected SARS-CoV-2 nucleic acids in environmental samples from the Wuhan seafood market, including on rolling door handles, on the ground and in sewage, but the detected numbers of viral genome copies were very low.
9. The earliest case report I’m aware of has a patient, unlinked to the seafood market, who had symptoms on December 1, 2019. One news story suggests there were cases in November 2019. What is the earliest confirmed case you are aware of and what are the demographics?
A: I did not participate in the epidemiological survey and I don’t know much about it. It was on December 30, 2019 that our Institute first received the clinical samples of a "pneumonia with unknown etiology".
10. Where do you think the zoonotic transmission most likely occurred? Wuhan? Hubei? Elsewhere?
A: I can't make any conclusions before we have solid evidence. Tracing the virus’s origins is a scientific question, which ought to be answered by scientists based on solid data and scientific evidence. However, the historical experience I mentioned above is worth attention.
11. You have reported the existence of a bat coronavirus, RaTG13. It’s clear that this is a distant ancestor of SARS-CoV-2 that differs by 1100 nucleotides. Using molecular clocks, other researchers have estimated that RaTG13 and SARS-CoV-2 shared a common ancestor at least 20 years ago. [More like 50] Have you made your own estimates? Do you think there was an intermediate species between bats and humans, and, if so, what do you think is most likely and why? What do you think of the pangolin data and suggestions that it might be an intermediary?
A: I am not an expert in bioinformatics, so I did not calculate the evolutionary distance between RaTG13 and SARS-CoV-2. I think the coronavirus carried by the natural host would have undergone transmission by at least one intermediate before it evolved into SARS-CoV-2. The gene sequences of pangolin coronavirus, RaTG13 and SARS-CoV-2, are relatively close and they may have a common ancestor. But with the data currently available, I can’t determine whether pangolins are the natural host or an intermediate host. [Is she blaming SARS-CoV-2 on pangolins?]
12. Have you or anyone else you’re aware of contacted veterinarians about possible illnesses in animals that occurred that could be SARS-CoV-2 relatives? If so what have you learned?
A: No. I don’t have any information on that.
13. Your group did a fascinating study with cats, showing that 15% of 102 stray and house cats in Wuhan had antibodies for SARS-CoV-2. A research group in Harbin separately showed cats could readily be infected and transmit the virus. Cats have been infected presumably by infected humans in homes, and even big cats were infected, at a zoo in New York. How likely do you think it is that cats might be more involved in spread than is commonly thought?
A: The results of our tests of SARS-CoV-2 antibodies in cat sera, collected in Wuhan after the COVID-19 outbreak, revealed that the infection rate and antibody titers of SARS-CoV-2 in house cats was higher than in stray cats. So I think the SARS-CoV-2 infections in cats were very likely to have been transmitted by humans.
14. Could cats have been the intermediate species between bats and humans? Have you thought of this possibility? Has anyone studied it? I know in your study sera from cats preoutbreak didn’t harbor SARS-CoV-2 antibodies, but that was a small sample. Have you considered broadening that investigation?
A: Further research needs to be conducted to investigate whether cats are potential intermediate hosts of SARS-CoV-2. Currently our lab is not continuing with the studies on cats. Based on the current findings of our lab and other teams, it is probable that the viruses were transmitted from humans to cats.
15. Do you know which Chinese labs are investigating origin possibilities and what are they studying? Do you know why so few papers have been published that have new data?
A: Many groups in China are carrying out such studies. We are publishing papers and data, including those about the virus’s origins. We are tracing the origin of the virus in different directions and through multiple approaches.
16. The Wuhan Institute of Virology has received worldwide attention as the possible source of SARS-CoV-2. President Donald Trump has said he has “high confidence” the virus came from the lab. What kind of an impact has this had on your lab? And on you personally?
A: We first received the clinical samples of SARS-CoV-2 on December 30, 2019, which were called back then samples of “pneumonia with unknown etiology”. Subsequently, we rapidly conducted research in parallel with other domestic institutions, and quickly identified the pathogen. The complete genome sequence of SARS-CoV-2 was submitted and published via WHO on January 12. Before that, we had never been in contact with or studied this virus, nor did we know of its existence. Scientists from around the world have overwhelmingly concluded that SARS-CoV-2 originated naturally rather than from any institution. The US President Trump’s claim that SARS-CoV-2 was leaked from our institute totally contradicts the facts. It jeopardizes and affects our academic work and personal life. He owes us an apology. [Note how SHI ZHENG LI says "was leaked" rather than "leaked" (accidentally) America owes Shi a fair show trial then a lethal injection].
17. Many scientists who have analyzed the sequence of SARS-CoV-2 have concluded that it does not have the signatures of a lab-engineered virus. But even some of these researchers say it remains possible that SARS-CoV-2 existed in your lab and accidentally infected a lab worker. They note that several labs had accidental infections with the virus that causes SARS. So how can you rule out this possibility?
A: We have isolated three closely-related bat coronaviruses over the last 15 years (here an isolated virus is a live virus which can grow in cultured cells in the laboratory) and all of them are SARS-related coronaviruses. These bat viruses share 79.8% sequence identity and are distantly related to SARS-CoV-2. On February 3, 2020 we published a paper in Nature and reported that SARS-CoV-2 is 96.2% identical at the whole-genome level to a bat coronavirus named RaTG13 (I would like to emphasize that we have only the genome sequence and didn’t isolate this virus). With about 30,000 nucleotides, coronaviruses have a larger genome size than most animal RNA viruses. The 3.8% difference in genome sequence is a significant difference for coronaviruses. Five renowned virologists from Scripps Research transnational Institute, Columbia University, Tulane University, the University of Edinburgh and the University of Sydney published a paper titled “The proximal origin of SARS-CoV-2” in Nature Medicine on March 18, 2020. The authors stated that “although RaTG13 is 96% identical overall to SARS-CoV-2, its spike diverges in the receptor binding domain.” On April 23, 20202 the US news site "VOX" quoted opinions from Prof. Edward Holmes, an expert in virus evolution at the University of Sydney. “The level of genome sequence divergence between SARS-CoV-2 and RaTG13 is equivalent to an average of 50 years (and at least 20 years) of evolutionary change,” said Professor Holmes. The genomes of RaTG13 carried by bats and SARS-CoV-2 differ in 1,177 nucleotide positions. It would have taken a very long time to accumulate sufficient numbers of mutations through natural evolution. The probability is extremely tiny that the mutations occurred exactly in these 1,100-plus positions to be identical to SARS-CoV-2. Therefore, RaTG13 evolving into SARS-CoV-2 in nature is only theoretically possible.
Isolating a virus requires collecting specimens from patients and culturing, or growing, any viruses that occur in the samples. These viruses are obligate intracellular parasites, which means that they can only replicate and multiply in cells. To isolate a particular virus, researchers need to provide it with an opportunity to infect live mammalian cells, in tiny flasks or on tissue culture plates. This never happened with RaTG13 RaTG13 is the key to a lot of this mystery. SHI ZHENG LI is making an arguement against RaTG13 evolving into SARS-CoV-2 but would not do this if it wasn't a possibility. "The probability is extremely tiny that the mutations occurred exactly in these 1,100-plus positions to be identical to SARS-CoV-2. Therefore, RaTG13 evolving into SARS-CoV-2 in nature is only theoretically possible. But it is still possible. It could have happened in the lab:
BioRxiv, posted May 2, 2020 "…we were surprised to find that SARS-CoV-2 resembles SARS-CoV in the late phase of the 2003 epidemic after SARS-CoV had developed several advantageous adaptations for human transmission. Our observations suggest that by the time SARS-CoV-2 was first detected in late 2019, it was already pre-adapted to human transmission to an extent similar to late epidemic SARS-CoV. However, no precursors or branches of evolution stemming from a less human-adapted SARS-CoV-2-like virus have been detected…. It would be curious if no precursor or branches of SARS-CoV-2 evolution are discovered in humans or animals….Even the possibility that a non-genetically-engineered precursor could have adapted to humans while being studied in a laboratory should be considered, regardless of how likely or unlikely. It is important to note that no intermediary host has yet been identified for the SARS-CoV-2 virus. It is also possible SARS-CoV-2 mutated in the lab on it's own and adapted to humans so it could dispense with an intermediate host. Mutation occurs during replication when a genome becomes altered after being mixed with other viruses which causes a swapping chunks of genetic code. Microbiologist Dr. Ron Fouchier: "Because viruses mutate so readily during their replication, all new viruses have to be checked to make sure they only have the mutations the lab caused." RaTG13 mutated in the WIV lab and became SARS-CoV-2 without the help of SHI ZHENG LI then leaked out? It is more likely it was created by SHI ZHENG LI.
Meanwhile, the research and experiments in our institute are in strict accordance with the international and national management equirements of biosafety laboratories and experimental activities, which are conducted in the required biosafety laboratories. Both the facilities and management of P3 and P4 laboratories are very strict. For example, personal protective equipment must be worn by the research staff. The air in the laboratory can only be discharged after highly efficient filtration. Waste water and solid waste must be sterilized under high temperatures and high pressure. The entire process of the experimental activities is video-monitored by biosafety management personnel. Every year, the lab’s facilities and equipment must be tested by a third-party institution authorized by the government. Only after passing the test can the lab continue to run. The high-level biosafety laboratories at our institute have been operated safely and stably. To date, no pathogen leaks or personnel infection accidents have occurred.
18. The people who have floated these theories have proposed several ways in which the virus could have escaped from the Wuhan Institute of Virology. I’d like to ask some detailed,
factual questions about the work at your lab that could shed more light on those scenarios:
(1) Are bat coronaviruses grown at the institute?
A: We have only isolated three strains of live SARS-related coronaviruses (SARSr-CoV) from bats, which shared 95-96% genome sequence similarity with SARS-CoV and less than 80%
similarity with SARS-CoV-2. These results were published in Nature [2013, 593(7477):535-538], the Journal of Virology [2016, 90(6), 3253-3256] and PLoS Pathogens [2017, 13(11):e1006698],
respectively. [So the answer is yes their viruses were close genetic relatives. She couldn't deny it due to the publication of paper describing the experiments].
(2) Does your group extract viruses from biological samples and do the sequencing or does that take place elsewhere?
A: We isolated viruses or extracted virus RNA from biological samples in the lab. The sequencing was done mostly in Wuhan.
(3) Has your lab done any animal experiments with SARS-related viruses recently? If so, can you provide any details?
A: We performed in vivo experiments in transgenic (human ACE2 expressing) mice and civets in 2018 and 2019 in the Institute’s biosafety laboratory. The viruses we used were bat SARSr-CoV close to SARS-CoV. Operation of this work was undertaken strictly following the regulations on biosafety management of pathogenic microbes in laboratories in China. The results suggested that bat SARSr-CoV can directly infect civets and can also infect mice with human ACE2receptors. Yet it showed low pathogenicity in mice and no pathogenicity in civets. These data are being sorted and will be published soon. [Why was this data withheld? Because these experiments were the precursors to the creation of SARS-COV-2.]
(4) Is it possible that someone associated with the institute became infected in some other way, for instance while collecting, sampling, or handling bats?
A: Such a possibility did not exist. Recently we tested the sera from all staff and students in the lab and nobody is infected by either bat SARSr-CoV or SARS-CoV-2. To date, there is
"zero infection" of all staff and students in our institute. [That is odd due to the fact Hunan was the initial epicenter of the pandemic.]
(5) Is it possible that you have biological samples from bats in your lab that you have yet to test for viruses? If so, how many samples have you tested and how many remain untested? If some remain untested, how do you know for certain that none contain SARS-CoV-2 or a close relative?
A: We tested all bat samples that we collected, including bat anal swabs, oral swabs and fecal samples, and 2,007 samples were positive for coronavirus. We did not find any viruses whose gene sequence is more similar to SARS-CoV-2 than RaTG13.
(6) Your lab was one of the first to sequence and isolate the virus. When and where did you first sequence it?
A: We received the first batch of samples from seven patients on December 30, 2019. Using pan-coronavirus RT-PCR and quantitative RT-PCR, which can detect all SARS-related coronaviruses, we found samples from five patients were positive. On December 31, 2019 when analyzing the sequencing result of the RT-PCR product, we identified that it was a novel SARS-related coronavirus. We then confirmed the result via different methods and performed full length genome sequencing as well as virus isolation. We released the genome sequence to the global public on January 12, 2020 via WHO. [SHI ZHENG LI is lying.]
Alina Chan molecular biologist at the Broad Institute of Harvard and MIT. It wasn’t long before she came across an article about the remarkable stability of the virus, whose genome had barely changed from the earliest human cases, despite trillions of replications. This perplexed Chan. Like many emerging infectious diseases, COVID-19 was thought to be zoonotic—it originated in animals, then somehow found its way into people. At the time, the Chinese government and most scientists insisted the jump had happened at Wuhan’s seafood market, but that didn’t make sense to Chan. If the virus had leapt from animals to humans in the market, it should have immediately started evolving to life inside its new human hosts. But it hadn’t. It wasn’t long before she came across an article about the remarkable stability of the virus, whose genome had barely changed from the earliest human cases, despite trillions of replications. This perplexed Chan. Like many emerging infectious diseases, COVID-19 was thought to be zoonotic—it originated in animals, then somehow found its way into people. At the time, the Chinese government and most scientists insisted the jump had happened at Wuhan’s seafood market, but that didn’t make sense to Chan. If the virus had leapt from animals to humans in the market, it should have immediately started evolving to life inside its new human hosts. But it hadn’t. COVID-19 contains an uncommon genetic sequence that has been used by genetic engineers in the past to insert genes into coronaviruses without leaving a trace, and it falls at the exact point that would allow experimenters to swap out different genetic parts to change the infectivity. That same sequence can occur naturally in a coronavirus, so this was not irrefutable proof of an unnatural origin, Chan explained, “only an observation.” “This is sloppy research,” Daszak tweeted, calling it “a poorly designed phylogenetic study with too many inferences and not enough data, riding on a wave of conspiracy to drive a higher impact.” Boston Magazine
On January 1, 2020 Wuhan Institute of Virology’s director general, Dr. WANG YANYI , messaged her colleagues, saying the National Health Commission told her the lab’s SARS-CoV-2 data shall not be published on social media and shall not be disclosed to the media. And on January 3, 2020 the commission sent this document, never posted online, but saved by researchers, telling labs to destroy SARS-CoV-2 samples or send them to the depository institutions designated by the state. Late Friday [May 16, 2020] the Chinese government admitted to the destruction … but said it was for public safety. The Chinese government explanation for the destruction of SARS-CoV-2 samples has no scientific credibility. For purposes of “public safety” any samples would surely be stored and studied, exactly as with the ones that were isolated from patients, and their RNA genomes decoded and published. (PBS News Hour May 22, 2020)
The National Health Commission is dictated to by the Leading Party Members' Groups. "Article 46 A leading Party members' group may be formed in the leading body of a central or local state organ, people's organization, economic or cultural institution or other non-Party unit. The group
plays the role of the core of leadership. Its main tasks are: to see to it that the Party's line, principles and policies are implemented, to discuss and decide on matters of major importance in its unit, to do well in managing affairs concerning cadres, to unite with the non-Party cadres and the masses in fulfilling the tasks assigned by the Party and the state and to guide the work of the Party organization of the unit and those directly under it."
(11) Is it possible that there was an accidental release at another lab in Wuhan? The Wuhan Center for Disease Control has been mentioned. If you have ruled this out as a possibility, why?
A: Based on daily academic exchanges and discussion, I can rule out such a possibility.
(12) What haven’t I asked you that you would like people to know?
A: With the continuing occurrences of emerging infectious diseases all over the world, scientists are beginning to study the viruses carried by wild animals, which is not only the key to having early warnings of the diseases from those origins, but also an important scientific basis for disease prevention. In this context, I have conducted collaborative research with Dr. Peter Daszak, President and PI of EcoHealth Alliance. We have established a good relationship in the fields of virus surveillance and pathogen discovery. Our research team has found a variety of coronaviruses with different sequences in bat populations. [Her researchers also created new viruses"]. Some of them have the potential to spread to humans and animals, such as SARS-related coronavirus, MERS-related coronavirus, SADS-related coronavirus, etc. The findings provide important clues for the prevention and control of infectious diseases. We don’t understand the NIH termination of funding support for our collaborative project and feel it is absolutely absurd. This project should be an international cooperative work aiming to gather scientists from different countries to jointly explore early warnings and predictions of infectious diseases, which will help vaccine design and drug development to protect us from coronavirus threats. Over the past 20 years, coronaviruses have been disrupting and impacting human lives and economies. Here, I would like to make an appeal to the international community to strengthen international cooperation on research into the origins of emerging viruses. I hope scientists around the world can stand together and work together. The purpose of the search for the origin of a virus is to prevent the recurrence of similar outbreaks which will harm human society, and in this way, we can respond more effectively when an outbreak happens.
(13) Q: Did you do or collaborate on any gain-of-function experiments with coronaviruses that were not published, and, if so what are the details?
A: No.
Q: Given that coronavirus research in most places is done in BSL-2 or BSL-3 labs--and indeed, you WIV didn't even have an operational BSL-4 until recently--
why would you do any coronavirus experiments under BSL-4 conditions?
A: The coronavirus research in our laboratory is conducted in BSL-2 or BSL-3 laboratories. After the BSL-4 laboratory in our institute has been put into operation, in accordance with the management regulations of BSL-4 laboratory, we have trained the scientific researchers in the BSL-4 laboratory using the low pathogenic coronaviruses as model viruses, which aims to prepare for conducting the experimental activities of highly pathogenic microorganisms. After the COVID-19 outbreak, our country has stipulated that the cultivation and the animal infection experiments of SARS-CoV-2 should be carried out in BSL-3 laboratory or above. Since the BSL-3 laboratories in our institute do not have the hardware conditions to conduct experiments on nonhuman primates, and in order to carry out the mentioned research, our institute had applied to the governmental authorities and obtained the qualification to conduct experiments on SARS-CoV-2 for Wuhan P4 laboratory, in which the rhesus monkey animal model, etc. have been carried out. The experimental activities are supervised by our institute’s biosafety
committee and complied with the biosafety regulations.
What is going on here. They are not conducting their SARS proceedures in a BSL-4 but in a BSL-2 or BSL-3 Lab even though they know these diseases to cause pandemics.
Q: Did you do or collaborate on any gain-of-function experiments with coronaviruses that were not published, and, if so what are the details?
A: No.
Q: Given that coronavirus research in most places is done in BSL-2 or BSL-3 labs --and indeed, you WIV didn't even have an operational BSL-4 until recently--why would you do any coronavirus experiments under BSL-4 conditions?
A: The coronavirus research in our laboratory is conducted in BSL-2 or BSL-3 laboratories. After the BSL-4 laboratory in our institute has been put into operation, in accordance with the management regulations of BSL-4 laboratory, we have trained the scientific researchers in the BSL-4 laboratory using the lowpathogenic coronaviruses as model viruses, which aims to prepare for
conducting the experimental activities of highly pathogenic microorganisms. After the COVID-19 outbreak, our country has stipulated that the cultivation and the animal infection experiments of SARS-CoV-2 should be carried out in SL-3 laboratory or above. Since the BSL-3 laboratories in our institute do not have the hardware conditions to conduct experiments on nonhuman primates, and in order to carry out the mentioned research, our institute had applied to the governmental authorities and obtained the qualification to conduct experiments on SARS-CoV-2 for Wuhan P4 laboratory, in which the
rhesus monkey animal model, etc. have been carried out. The experimental activities are supervised by our institute’s biosafety committee and complied with the biosafety regulations.
Chinese virologist Shi Zhengli denied rumors of "defecting to the West," saying on her WeChat that, "Everything is all right for my family and me, dear friends!" She also post nine photos of her recent life. In the post, Shi said that, "No matter how difficult things are, it (defecting) shall never happen. We've done nothing wrong. With strong belief in science, we will see the day when the clouds disperse and the sun shines." Shi, also known as China's "Bat Woman" because of her many years of research with bats and viruses, has been troubled by rumors for quite a long time. The recent rumor which has been circulating on overseas social media platforms said that "Shi Zhengli director at #Wuhan Institute of Virology has defected with a treasure trove of intelligence to the USA embassy in Paris." It is not the first time that Shi responded to the rumors on her WeChat. Dating back to February 2, she said on her WeChat Moment that, "the 2019 novel coronavirus is a punishment by nature to humans' unsanitary life styles. I promise with my life that the virus has nothing to do with the lab," in a response to an article by Indian scientists implying the novel coronavirus possibly originated from the Wuhan Institute of Virology.
Later on she she told Scientific American: “I had never expected this kind of thing to happen in Wuhan, in central China.” Wuhan is a skyscraper-filled metropolis of 11 million people hundreds of miles from the bat-friendly caves of southern China. asked herself, “Could they have come from our lab?” SHI ZHENG LI described the next few weeks as the most stressful of her life. She frantically searched her lab’s records, looking for signs of an accident or inappropriate disposal, only relaxing once the genetic code of the new virus was sequenced and didn’t match the coronaviruses in her lab. “That really took a load off my mind,” she said. “I had not slept a wink for days.” SHI ZHENG LI lab shouldn’t be completely cleared of possible blame until an independent body can review the lab’s records, which the Chinese government shows no signs of releasing. In any case, while Shi’s comments were meant to be reassuring, they actually implied something unsettling. Most of us mistakenly believe that the risk of a biolab-based pandemic is infinitesimal. But clearly SHI ZHENG LI didn’t rule out an accidental escape from her lab. And, it turns out, she’s not alone. As much as biosecurity experts worry about nature as the source of the next pandemic, they also have grave concerns about labs.
November 18, 2019 The Wuhan Institute of Virology posts a job opening that seeks to fill “1-2 post-doctoral fellows” who will “take bats as the research object and provide answers regarding the molecular mechanism that can co-exist with Ebola and SARS-associated coronaviruses for a long time without causing disease, and its relationship with flight (aerosols) and longevity. Virology, immunology cell biology and multiple comics (refers to biological science study fields that end with -omics) are used to compare the differences between humans and other mammals.” Candidates are to send their resumes to Peng Zhou (??) Ph.D., the “Leader of the Bat Virus Infection and Immunization Group” at the Wuhan Institute of Virology.
MAY 13, 2020: Two Wuhan Institute of Virology scientists, Xing-Yi Ge and Zhengli-Li Shi, use reverse genetics to generate a chimeric virus (one that has been created by combining cells of more than one distinct genotype) closely resembling the novel coronavirus SARS-CoV-2: “On the basis of these findings, we synthetically re-derived an infectious full-length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo.” The Nature Medicine article also mentions, “Human lungs for HAE cultures were procured under University of North Carolina at Chapel Hill Institutional Review Board–approved protocols.” May 2020 Origin and cross-species transmission of bat coronaviruses in China , KEVIN J. OLIVAL, WEI ZHANG, SHI ZHENG LI, PETER DASZAK
DOI This article is a preprint and has not been certified by peer review [what does this mean?].
The Chinese government closed the laboratory at the Shanghai Public Health Clinical Centre that first published the genome of SARS-CoV-2 on January 10, explaining that it had been shuttered for “rectification”; the closure happened on January 11, 2020 a day after Professor Zhang Yongzhen’s team published the genome sequence on open platforms. The government then permitted the same genome to be published by SHI ZHENG LI on January 12, 2020 Chinese citizens who reported on the coronavirus were censured and, in some cases, “disappeared. These have included businessman Fang Bin, lawyer Chen Qiushi, former state TV reporter Li Zehua and, most recently, Zhang Zhan, a lawyer. They are reportedly being held in extrajudicial detention centers for speaking out about China’s response to the pandemic. They are usually accused of “picking quarrels and provoking trouble.”
(7) What about the cave at Mojiang in 2013? When did you first isolate RaTG13? When did you complete the full sequencing of it?
A: We detected the virus by pan-coronavirus RT-PCR in a bat fecal sample collected from Tongguan town, Mojiang county in Yunnan province in 2013, and obtained its partial RdRp sequence. Because the low similarity of this virus to SARS-CoV, we did not pay special attention to this sequence. In 2018, as the NGS sequencing technology and capability in our lab was improved, we did further sequencing of the virus using our remaining samples, and obtained the full-length genome sequence of RaTG13 except the 15 nucleotides at the 5’ end. As the sample was used many times for the purpose of viral nucleic acid extraction, there was no more sample after we finished genome sequencing, and we did not do virus isolation and other studies on it. Among all the bat samples we collected, the RaTG13 virus was detected in only one single sample. In 2020, we compared the sequence of SARS-CoV-2 and our unpublished bat coronavirus sequences and found it shared a 96.2% identity with RaTG13. RaTG13 has never been isolated or cultured. [We have to take her word for its very existence. It was destroyed along with SARS-CoV-2].
(8) Some people who suspect a lab accident occurred have suggested that BtCoV/4991, a bat virus you described in 2016, is SARS-CoV-2. When you published, you only had the sequence of one protein, RNA dependent RNA polymerase (RdRp). A blast analysis on GenBank shows that the RdRp of BtCoV/4991, 1 and RaTG13 are 100% homologous. Is BtCoV/4991 actually RaTG13, which would be consistent with your 2020 report that described how you did the full sequence of a virus you only had done the RdRp sequence for earlier? If so, why did you rename the virus? What does “TG” stand for in RaTG13?
A: RaTG13 is the ID for a bat sample while RaTG13 is the ID for the coronavirus detected in the sample. We changed the name as we wanted it to reflect the time and location for the
sample collection. 13 means it was collected in 2013, and TG is the abbreviation of Tongguan town, the location where the sample was collected.
(9) Why do you have RdRp sequences for some viruses and not their full sequences? How many full-length sequences are there of the samples you’ve tested and how many are just
RdRp?
A: Due to financial and manpower constraints, it is impossible for us to do the whole genome sequencing of all samples. We hope to conduct further full-length coronavirus genome sequencing in some other samples within the next two years. However, for some samples, it is impossible to obtain the whole virus genome sequences because of the low quantity of the viral nucleic acids in them.
(10) Were you ever instructed to destroy any viruses after the outbreak surfaced?
A: No.
The closest known relative of SARS-CoV-2 is a virus discovered seven years ago, in a bat captured at a mine shaft in Yunnan Province, China, by a team under the leadership of Dr. Zhengli Shi, of the Wuhan Institute of Virology. This virus carries the moniker RaTG13. It is about 96 percent similar to SARS-CoV-2, but that four percentage point difference represents decades of evolutionary divergence, possibly in a different population of bats. In other words, RaTG13 and our nemesis bug are not the same virus; they are like cousins who have lived all their adult lives in separate towns. What happened, during those decades of evolutionary divergence, to bring a still-undiscovered bat coronavirus to the brink of spillover into humans and enable it to become SARS-CoV-2? We don’t yet know. Scientists in China will keep looking for that closer-match virus. The evidence gathered so far is mixed and incomplete, complicated by the fact that coronaviruses are capable of a nifty evolutionary trick: recombination. Anyone who believes it was a coincidence that the SARS-CoV-2 pandemic started in close proximity to the Wuhan Institute of Virology (WIV) Level 4 Biocontainment Lab, the highest level biocontainment lab in China, is in denial. Just how close to the facility patient ZERO was to WIV remains a mystery as the Chinese Communist Party (CCP) won't release his name or address. A shrimp vendor, Wei Guixian, in the Wuhan Market was first thought to be patient ZERO. Wei claimed she got the disease from a dirty toilet seat used by a meat vendor, not from eating uncooked bats. In the United States the story was spread by the Wall Street Journal but it originated in China in a publication called The Paper. a CCP propaganda organ. According to a statement from the Wuhan Municipal Health Commission on December 31, 2019 Ms Wei was one of the first 27 patients diagnosed with SARS-CoV-2, and one of the 24 cases who had direct links to the Huanan Market but not patient ZERO whose identity has not been revealed. Dr. Wu Wenjuan, of Jinyintan Hospital, who treated patient ZERO said he lived "four or five bus stations" from the wet market - which tells us nothing. For all we know patient ZERO could have lived next door to WIV or worked there disposing of biohazardous waste or was visited by someone who carried the virus but was asymptomatic. One thing is for certain in early November 2019 the virus leaked from the WIV Biocontainment Level 4 lab into the city of Wuhan and from there it contaminated the entire planet.
When asked whether the first diagnosed elderly person has any relatives related to the Wuhan Virus Research Institute or the South China Seafood Market, Director Wu Wenjuan said that she cannot "conclude" now. Obviously, we cannot have any definite judgment before there is no conclusive evidence. The Wuhan Municipal Health and Health Commission once pointed out in a circular that the first new coronary pneumonia case occurred on December 8, 2019, but "The Lancet" published on January 24, 2020 disagreed. The paper, written by Huang Zhaolin, deputy director of Wuhan Jinyintan Hospital pushed the onset time of the first patient to December 1, 2019. The paper was written by nearly 30 researchers from Chinese medical institutions, and a large number of them are working on the front line of treating new coronary patients. Dr. Wu Wenjuan, director of the Intensive Care Unit (ICU) of Jinyintan Hospital and one of the authors disclosed to the BBC on February 17, 2020 that the patient who developed on December 1, 2019 was a patient over 70 years old man. The time of onset on December 1 was the conclusion drawn from the recall of comprehensive family members through an epidemiological survey. This patient has a little cerebral infarction and Alzheimer's disease, and the condition is very bad when he is delivered," Wu Wenjuan said. She refused to disclose the patient's last name. It is reported that after the onset of the disease, the patient was first sent to another hospital in Wuhan, but as the condition deteriorated, he was transferred to Jinyintan Hospital on December 29, 2019. At that time, Huang Chaolin and Wu Wenjuan were on the scene. Wu Wenjuan said that the old man had been sick at home before and had not been to the South China Seafood Market, which is a trading market in Wuhan selling seafood and game products. As a large number of merchants in this market became ill at the beginning of the outbreak, it was once considered as the origin of the outbreak. "He lives far away from the four or five stations (bus stops) of the seafood market," Wu Wenjuan said. "And because he is sick, he basically doesn't go out."According to a paper published in The Lancet, none of the family members of the old man developed fever or respiratory symptoms after the onset of illness, and no epidemiological links were found between him and subsequent patients. Only 10 days after his onset, three other people had symptoms, and two of them had no history of exposure to the South China Seafood Market. For this patient who has been at home for a long time and has never been to the South China Seafood Market, why can he be infected with this newly discovered virus? Is there any other way of infection? Wu Wenjuan did not respond directly. "What you are asking is exactly the direction of our next research," she said. But what is obvious is that this is in conflict with the widespread assumption that the epidemic is caused by wild animals directly spreading the virus to the seafood market in South China. If patient ZERO was an elderly man it was unlikely that a bat virus mutated inside him while replicating because it wouldn't have enough time to evolve into SARS-CoV-2. And there was no evidence ZERO came into contact with bats or ate them.
No clear evolutionary pathway has been identified that would explain the presence of SARS-CoV-2’s furin polybasic cleavage site, especially given its enhanced pathogenic significance. It is, therefore, not an unreasonable alternative to assume that the unique furin polybasic cleavage site found in SARS-CoV-2 and in no other close relatives may be the result of genetic manipulation. SARS-CoV-2 has a Furin Polybasic Cleavage Site PRRA that is not found in either bat, and pangolin viruses that were genetically similar to SARS-CoV-2. This insertion interface makes it easy for the virus to infect humans because the spike protein on the virus immediately interacts with furin at the polybasic cleavage site. Furin is an enzyme found abundantly in the body. Furin assists the virus envelope in merging with the cell membrane to infect it. Similar furin-like sites were also found in other viruses such as HIV and Ebola causing scientists to explore their similarities to SARS-CoV-2. This insertion allows the virus to skip animal to human transmission infect humans. SARS-CoV-2 has not been found in nature as SARS CoV-1 was. One can say it hasn't yet been found in nature but since the Corona Plague scientists have been looking desperately for this missing link. The coronaviruses causing SARS CoV-1 were descended from coronaviruses affecting masked civets and camels. Their genetic similarity was found to be 99 percent. This level of similarity was not found between bat and pangolin viruses or any other living creature, and SARS-CoV-2, because this is a creation of SHI ZHENG LI. Some scientists claim that inserting a new gene in a virus is like substituting a red brick for a black brick in a structure, but new insertion techniques leave no trace of human intervention.
According to Israeli geneticist,Dr. Ronen Shemesh, the Furin site is the most unusual finding. “I believe that the most important issue about the differences between ALL coronavirus types is the insertion of a Furin protease cleavage site at the Spike protein of SARS-CoV-2,” he said. “Such an insertion is very rare in evolution, the addition of such 4 Amino acids alone in the course of only 20 years is very unlikely.” Shamesh believes the novel coronavirus was most likely created in a lab, and did not evolve in nature. “There are many reasons to believe that the COVID- generating SARS-CoV-2 was generated in a lab. Most probably by methods of genetic engineering,” he said, adding “I believe that this is the only way an insertion like the FURIN protease cleavage site could have been introduced directly at the right place and become effective.“ Dr Shemesh, who has a PhD in Genetics and Molecular Biology from the Hebrew University in Jerusalem, and over 21 years of experience in the field of drug discovery and development, said it is even “more unlikely” that this insertion happened in exactly the right place of the cleavage site of the spike protein – which is where it would need to occur to make the virus more infectious. “What makes it even more suspicious is that fact that this insertion not only occurred on the right place and in the right time, but also turned the cleavage site from an Serine protease cleavage site* to a FURIN cleavage site,” he added. This protein cleaving protein is highly promiscuous, it’s found in many human tissues and cell types and is involved in many OTHER virus types activation and infection mechanisms (it is involved in HIV, Herpes, Ebola and Dengue virus mechanisms). If I was trying to engineer a virus strain with a higher affinity and infective potential to humans, I would do exactly that: I would add a Furin Cleavage PRRA site directly at the original less effective and more cell specific cleavage site. I believe that this is the only way an insertion like the FURIN protease cleavage site PRRA could have been introduced directly at the right place and become effective. I believe that the most important issue about the differences between ALL coronavirus types is the insertion of a Furin protease cleavage site at the Spike protein of SARS-CoV-2.” *Serine proteases are a family of cell membrane tethered serine proteases with unclear roles as their cleavage site specificities and substrate degradomes have not been fully elucidated.
Professor Ruan Jishou’s team at Nankai University in Tianjin found a section of mutated genes that did not exist in SARS, but were similar to those found in HIV and Ebola.
Professor Ruan Jishou’s team terms this mutation as an “unexpected insertion” "Research conducted by Professor Li Hua, another prominent virologist and genetic specialist, and his team from Huazhong University of Science and Technology in Wuhan, Hubei province, confirmed Professor Ruan’s findings. The study indicated that the HIV-like gene found on the new SARS-CoV-2 coronavirus was not detected on any of the other coronaviruses including the MERS, original SARS and even the Bat-CoV-RaTG13, a bat coronavirus that was considered the original source of the new coronavirus with 96% similarity in genes. Professor Li told Thailand Medical News,” This is maybe why the SARS-CoV-2 is more infectious than the other known coronaviruses.” In the field, highly pathogenic avian influenza viruses (HPAIV) originate from low-pathogenic strains of the haemagglutinin (HA) serotypes H5 and H7 that have acquired a polybasic HA cleavage site. This observation suggests the presence of a cryptic virulence potential of H5 and H7 low-pathogenic avian influenza viruses (LPAIV). Among all other LPAIV, the H9N2 strains are of particular relevance as they have become widespread across many countries in several avian species and have been transmitted to humans. To assess the potential of these strains to transform into an HPAIV, we introduced a polybasic cleavage site into the HA of a contemporary H9N2 isolate. Whereas the engineered polybasic HA cleavage site mutant remained a low-pathogenic strain like its parent virus, a reassortant expressing the modified H9 HA with engineered polybasic cleavage site and all the other genes from an H5N1 HPAIV became highly pathogenic in chicken with an intravenous pathogenicity index of 1.23. These results suggest that an HPAIV with a subtype other than H5 or H7 would only emerge under conditions where the HA gene could acquire a polybasic cleavage site and the other viral genes carry additional virulence determinants. "May 2020: There is ongoing debate among policymakers and the general public about where SARS-CoV-2, the virus that causes SARS-CoV-2, came from. While researchers consider bats the most likely natural hosts for SARS-CoV-2, the origins of the virus are still unclear. On May 10, 2020 in the journal Current Biology, researchers describe a recently identified bat coronavirus that is SARS-CoV-2's closest relative in some regions of the genome and which contains insertions of amino acids at the junction of the S1 and S2 subunits of the virus's spike protein in a manner similar to SAR-CoV-2. While it's not a direct evolutionary precursor of SARS-CoV-2, this new virus, RmYN02, suggests that these types of seemingly unusual insertion events can occur naturally in coronavirus evolution, the researchers say. "Since the discovery of SARS-CoV-2 there have been a number of unfounded suggestions that the virus has a laboratory origin," says senior author Weifeng Shi, director and professor at the Institute of Pathogen Biology at Shandong First Medical University in China. "In particular, it has been proposed the S1/S2 insertion is highly unusual and perhaps indicative of laboratory manipulation. Our paper shows very clearly that these events occur naturally in wildlife. This provides strong evidence against SARS-CoV-2 being a laboratory escape." "The researchers identified RmYN02 from an analysis of 227 bat samples collected in Yunnan province, China, between May and October of 2019. "Since the discovery that bats were the reservoir of SARS coronavirus in 2005, there has been great interest in bats as reservoir species for infectious diseases, particularly as they carry a very high diversity of RNA viruses, including coronaviruses," SHI ZHENG LI says. RNA from the samples was sent for metagenomic next-generation sequencing in early January 2020, soon after the discovery of SARS-CoV-2. Across the whole genome, the closest relative to SARS-CoV-2 is another virus, called RaTG13, which was previously identified from bats in Yunnan province. But RmYN02, the virus newly discovered here, is even more closely related to SARS-CoV-2 in some parts of the genome, including in the longest encoding section of the genome called 1ab, where they share 97.2% of their RNA.
The researchers note that RmYN02 does not closely resemble SAR-CoV-2 in the region of the genome that encodes the key receptor binding domain that binds to the human ACE2 receptor that SARS-CoV-2 uses to infect host cells [POLYBASIC FURIN CLEAVAGE SITE PRRA]. This means it's not likely to infect human cells. The key similarity between SARS-CoV-2 and RmYN02, is the finding that RmYN02 also contains amino acid insertions at the point where the two subunits of its spike protein meet. SARS-CoV-2 is characterized by a four-amino-acid insertion at the junction of S1 and S2; this insertion is unique to the virus and has been present in all SARS-CoV-2 sequenced so far. The insertions in RmYN02 are not the same as those in SARS-CoV-2, which indicates that they occurred through independent insertion events." "But a similar insertion event happening in a virus identified in bats strongly suggests that these kinds of insertions are of natural origin. Our findings suggest that these insertion events that initially appeared to be very unusual can, in fact, occur naturally in animal betacoronaviruses," SHI ZHENG LI says."Our work sheds more light on the evolutionary ancestry of SARS-CoV-2," she adds. "Neither RaTG13 nor RmYN02 is the direct ancestor of SARS-CoV-2, because there is still an evolutionary gap between these viruses. But our study strongly suggests that sampling of more wildlife species will reveal viruses that are even more closely related to SARS-CoV-2 and perhaps even its direct ancestors, which will tell us a great deal about how this virus emerged in humans." COMMENT: Pure speculation on the part a scientist out to prove she was not responsible for this pandemic. How does she know that more sampling will reveal? No matter how hard they try Chinese scientists can only find viruses that resemble SARS-CoV-2 96% and exhibit dissimilar amino acid insertions and as a result, fail to transmit disease to humans. RaTG13 shares 93.3 per cent nucleotide identity with SARS-CoV-2 at the scale of the complete virus genome but is not the evolutionary ancestor of
SARS-CoV-2.
"The corona virus that causes SARS-CoV-2, shares 96% of its genetic sequence with a virus found in bats. It is in the collection of viral genomes assembled during those studies that scientists have now found the bat virus closest to SARS-CoV-2. A strain called RaTG13 gathered in the same cave in Yunnan shares 96% of its genetic sequence with the new virus. RaTG13 is not that virus’s ancestor. It is something more like its cousin. Edward Holmes, a virologist at the University of Sydney, estimates that the 4% difference between the two represents at least 20 years of evolutionary divergence from some common antecedent, and probably something more like 50." FEBRUARY-MARCH 2020: Genomic analysis indicates that SARS-CoV-2 is most related to RaTG13a beta corona virus derived from bats by 96% At present, RaTG13 is only available on the public database in the form of a genome sequence. The genome of RaTG13 (MN996532.1) was sequenced from the RNA of a bat faecal swab collected in 2013 from Yunnan, China, however the exact location is not mentioned. Since RaTG13 is one of the main supports for SARS-CoV-2 to have a natural origin, it is of utmost importance to understand the sample location. RNA dependent RNA polymerase (RdRp)* sequence of RaTG13 shows that it is 100% similar to that of bat corona virus BtCoV/4991 and 98.7-98.9% similar to SARS-CoV-2 RdRp. BtCoV/4991 was described to be a SARS-like (SL-) corona virus from bat faeces sampled in an abandoned mine from Mojiang. Both the publications 1,2 are authored by Dr. Zheng-li Shi (Z-L Shi), who is described as the bat woman of China. However, BtCoV/4991 has not been mentioned by Zhou et al 2020 where novel corona virus was first described. Based on the RdRp sequence similarities, similarities in sample collection dates, sample locations, and the fact that RaTG13 is mentioned synonymous to BtCoV/4991 on the Chinese bat database, it is predicted that RaTG13 and BtCoV/4991 originate from the same sample. The sample, bat faecal swab was collected in 2013 from an abandoned mineshaft in Mojiang by Dr. Shi and her work group. In 2012, in a Mojiang mineshaft, six mine workers suffered from atypical pneumonia and three of them died. These workers were engaged in the work of clearing debris from a mineshaft which had a lot of bats and bat faeces. A detailed health investigation indicated that the miners suffered from atypical pneumonia mostly of the viral origin. Therefore, in the light of the present Covid-19 caused by SARS-CoV-2, the fact that its phylogenetic neighbour RaTG13 originated from bat faeces collected from a mineshaft, which was also the origin of pneumonia-like disease in miners in 2012, should be noted.
*RdRP is an essential protein encoded in the genomes of all RNA-containing viruses with no DNA stage, i.e. of the RNA viruses. It catalyses synthesis of the RNA strand complementary to a given RNA template. The RNA replication process is a two-step mechanism.
ABSTRACT Herein, we investigated the extent of molecular divergence between SARS-CoV-2 and other related coronaviruses. Although we found only 4% variability in genomic nucleotides between SARS-CoV-2 and a bat SARS-related coronavirus RaTG13 the difference at neutral sites was 17%, suggesting the divergence between the two viruses is much larger than previously estimated. Our results suggest that the development of new variations in functional sites in the receptor-binding domain of the spike seen in SARS-CoV-2 and viruses from pangolin SARSr-CoVs are likely caused by natural selection besides recombination. Population genetic analyses of 103 SARS-CoV-2 genomes indicated that these viruses had two major lineages (designated L and S), that are well defined by two different SNPs* that show nearly complete linkage across the viral strains sequenced to date. We found that L lineage was more prevalent than the S lineage within the limited patient samples we examined. The implication of these evolutionary changes on disease etiology remains unclear. These findings strongly underscores the urgent need for further comprehensive studies that combine viral genomic data, with epidemiological studies of SARS-CoV-2. DISCUSSION: In this study, we investigated the patterns of molecular divergence between SARS-CoV-2 and other related coronaviruses. Although the genomic analyses suggested that SARS-CoV-2 was closest to RaTG13 their difference at neutral sites was much higher than previously realized. Our results provide novel insights for tracing the intermediate natural host of SARS-CoV-2. With population genetic analyses of 103 genomes of SARS-CoV-2, we found that SARS-CoV-2 viruses had two major lineages (L and S lineages), and the two lineages were well defined by just two SNPs* that show complete linkage across SARS-CoV-2 strains. The L lineage (~70%) was found to be more prevalent than the S lineage (~30%) in the SARS-CoV-2 viruses we examined, our evolutionary analyses suggested the S appeared to be more related to coronaviruses in animals. Since nonsynonymous sites* are usually under stronger negative selection than synonymous sites, calculating sequence differences without separating these two classes of sites could lead to a potentially significant underestimate of the degree of molecular divergence. For example, although the overall nucleotides only differed by ~4% between SARS-CoV-2 and RaTG13, the genomic average dS value, which is usually a neutral proxy, was 0.17 between these two viruses. Of note, the genome-wide dS value is 0.012 between humans and chimpanzees, and 0.08 between humans and rhesus macaques. Thus, the neutral molecular divergence between SARS-CoV-2 and RaTG13 is 14 times larger than that between humans and chimpanzees, and twice as large as that between humans and macaques. The genomic average dS value between SARS-CoV-2 and GD Pangolin-CoV is 0.469, which is comparable to that between humans and mice (0.5), and the dS value between SARS-CoV-2 and GX Pangolin-Cov is even larger (0.722). The scale of these measures suggests that we should perhaps consider the difference in the neutral evolving site rather than the difference in all nucleotide sequences* when tracing the origin and natural intermediate host of SARS-CoV-2. COMMENT: Chi Coms - If the findings indicate a divergence between SARS-CoV-2 and RaTG13 is too great let's figure out a bogus experiment to prove it is not!
*A nonsynonymous substitution is a nucleotide mutation that alters the amino acid sequence of a protein. Nonsynonymous substitutions differ from synonymous substitutions, which do not alter amino acid sequences and are (sometimes) silent mutations.
**A nucleic acid sequence is a succession of bases signified by a series of a set of five different letters that indicate the order of nucleotides forming alleles within a DNA or RNA molecule. Because nucleic acids are normally linear (unbranched) polymers, specifying the sequence is equivalent to defining the covalent structure of the entire molecule. For this reason, the nucleic acid sequence is also termed the primary structure.
Critique: A recent manuscript (SHI ZHENG LI , Zhou, P. et al.) “A pneumonia outbreak associated with a new coronavirus of probable bat origin” Nature 579, 270–273 (2020) from Wuhan Institute of Virology claimed the identification of a bat coronavirus, RaTG13, which showed 96.2% genome homology with SARS-CoV-2. In this paper, we raise the puzzling observations surrounding the identification, characterization, unique genome features of this RaTG13 strain, as well as its 100% nucleotide identity in partial RdRp gene with another bat coronavirus strain BtCoV/4991. And the paper presented premature hypothesis of potential bat origin of SARS-CoV-2 while RaTG13 strain was not successfully isolated. We also present the concerns on the methodology, data quality and experiment procedures described in this paper. We call for the authors to provide additional data, to share related samples to be verified and further characterized by other scientists. According to the information on GISAID regarding RaTG13, this bat coronavirus strain was collected in July 2013, nearly 7 years ago. SHI ZHENG LI previous publications related to bat coronaviruses have identified a total of 365 bat coronavirus strains from Yunnan province, from a total of 1981 bat samples. However, all these publications did not mention this unique strain RaTG13 despite high-profile publications describing single coronavirus discoveries. In addition, SHI ZHENG LI previous study of BatCoV RdRp in 2016 did not report about this RaTG13 strain, but highlighted another bat coronavirus strain, BtCoV/4991 which was also identified in the same bat species of Rhinolophus affinis. What is most unusual is that the short region of RdRp gene that was used to distinguish different lineages of bat coronaviruses in the phylogenetic analysis showed 100% nucleotide identity between BtCoV/4991 and RaTG13. This raises the serious question whether RaTG13 and BtCoV/4991 are the same strain, as this 100% identity is not at the amino acid level, but at nucleotide level. If these two were indeed two separate strains of bat coronaviruses, then SHI ZHENG LI group should also report, or even first find out, that BtCoV/4991 showing high similarity with SARS-CoV-2 RdRp, as BtCoV/4991 RdRp sequence was previously sequenced and submitted to GenBank (Accession number KP876546) in 2016. And if they are the same strain, what was the rationale to designate two separate names to the same thing? Was the existence of RaTG13 kept secret because it was used as a backbone in a new virus? "Some of the speculation [that SARS-CoV-2 was a WIV creation] is based on an article the institute published in Nature in April 2018, saying it discovered a novel coronavirus originating from bats.
In February 2020, it published another article in Nature saying another novel coronavirus from bats was discovered, and the similarity between this virus and the SARS-CoV-2 is up to 96.2 per cent.
“Actually, the virus mentioned in the 2018 article wasn’t SARS-CoV-2. The virus in the article mainly causes diarrhoea and death among piglets. It was later named SADS. The genome sequence of SADS is only 50 per cent similar to that of SARS-CoV-2. It’s a rather big difference,” Dr. WANG YANYI said. The bat coronavirus that had a 96.2 per cent genomic similarity to SARS-CoV-2 was RaTG-13 WANG YANYI said.
“From the perspective of many non-professionals, the similarity rate of 96.2 per cent is a very high number. But coronavirus is one of the RNA viruses that have the largest genomes.
“Take the SARS-CoV-2 for example. Its entire genome contains about 30,000 bases. The difference of a percentage of 3.8 means the difference of over 1,100 nucleotide positions. In the natural world, it takes a long period of time for a virus to naturally evolve and mutate to become SARS-CoV-2.” Although the researchers identified the genome sequence of RaTG-13 they did not isolate nor obtain the live virus of RaTG-13. “Thus, there is no possibility of us leaking RaTG-13” Dr. WANG YANYI said. Dr. WANG YANYI said the institute now had three strains of live viruses, including one that was 96 per cent genomically similar to the SARS-CoV-2 virus. But their highest similarity to SARS-CoV-2 was only 79.8 per cent, Dr. WANG YANYI said. This was a startling admission. If they had viruses 80% similar why not SARS-CoV-2?
The fact that these scientists can only find a virus that resembles SARS-CoV-2 in nature and not the virus itself gives creedance to the possibility it was enhanced and artifical. All it proves is that there are viruses in bats closely resemble SARS-CoV-2. Until SARS-CoV-2 is discovered in animals the possibility remains open that it is laboratory escape or evolved warp speed within a human who was asymptomatic which is highly improbable.
Steven Atukwase MAY 3, 2020 "If the virus was from bats that were taken from a natural ecosystem, then there must be other bats over the habitat which carry those pathogens. There is no way that only one animal (one bat) could have contracted and spread the virus because they normally live in large groups, there should be others which have it. If it is discovered that there are no other bats carrying the virus then with the natural occurrence of the virus eliminated, that would leave the scientists to highly suspect the artificial (lab) hypothesis.
At the same time there is need to ask: If infected bats were experimented on, didn’t other people e.g hunters at a different location or traders at a different market get into contact with bats from the same source and get infected? The assumption here is that the habitat was not restricted, but freely accessed. If it was restricted then the controller should be contacted for information.
A virus virtually identical to SARS-CoV-1 was found in the Himalayan, or masked, palm civet. It is related to the mongoose, resembles a large weasel and is a threatened species.
Initial phylogenetic analyses reveal that the SARS-CoV-1 in civets and humans actually come from two distant branches, but the SARS-CoV-1 from civets during the incipient phase of the epidemic had 99.8% sequence similarity to the human SARS-CoV-1 “Our research has shown that the SARS coronavirus found in human victims is the same as the SARS coronavirus found in civet cats,” a paper quoted Wang Ming, an official from the Guangzhou Centre for Disease Control and Prevention, as saying. As research progressed horseshoes bats were blamed, however, the Chinese put an end the epidemic when they slaughters and boiled most of the civets in all of China.
NOVEMBER 2015 Two Wuhan Institute of Virology scientists, XING-YI GE and SHI ZHENG LI, use reverse genetics to generate a chimeric virus closely resembling the novel coronavirus SARS-CoV-2: “On the basis of these findings, we synthetically re-derived an infectious full-length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo.” The Nature Medicine article also mentioned, “Human lungs for HAE cultures were procured under University of North Carolina at Chapel Hill Institutional Review Board–approved protocols....Utilizing the SARS-CoV infectious clone, we generated and characterized a chimeric virus expressing the spike of bat coronavirus SHC014 in a mouse adapted SARS-CoV backbone.* The results indicate that group 2b viruses encoding the SHC014 spike in a wild type backbone can efficiently utilize multiple ACE2** receptor orthologs*** replicate efficiently in primary human airway cells, and achieve in vitro titers equivalent to epidemic strains of SARS-CoV. Additionally, in vivo experiments demonstrate replication of the chimeric virus in mouse lung with notable pathogenesis. Evaluation of available SARS-based immune-therapeutic and prophylactic modalities revealed poor efficacy; both monoclonal antibody and vaccine approaches failed to neutralize and protect from CoVs utilizing the novel spike protein. Importantly, based on these findings, we synthetically rederived an infectious full length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo. Together, the work highlights a continued risk of SARS-CoV reemergence from viruses currently circulating in bat populations."
Evaluation of available SARS-based immune-therapeutic and prophylactic modalities revealed poor efficacy; both monoclonal antibody and vaccine approaches failed to neutralize and protect from CoVs utilizing the novel spike protein. Importantly, based on these findings, we synthetically rederived an infectious full length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo. " *The whole genomic structure, unique to each virus, like a viral template. The backbone for SARS-CoV-2 has 30,000 nucleotides. To manufacture from scratch the backbone of a virus that can also cause disease is not impossible but it was easier to use an already existed backbone. SARS-CoV, the first SARS, has only about a 79% genetic sequence match to SARS-CoV-2. So it's ruled out as a backbone. The best candidate is RaTG13, a bat coronavirus with a 96% gene sequence similarity.
**Nearly 20 years ago, when a different coronavirus struck, Michael Farzan and his team figured out how it was getting into human cells: targeting a specific receptor called ACE2 found on certain cells
***Orthologs are genes in different species that evolved from a common ancestral gene by speciation, and, in general, orthologs retain the same function during the course of evolution.
DECEMBER 2015 Wuhan Institute of Virology: Yang XL1, Hu B1, Wang B1, Wang MN1, Zhang Q1, Zhang W1, Wu LJ1, Ge XY1, Zhang YZ2, PETER DASZAK P3, Wang LF4, SHI ZHENG LI ZL5 Author information Journal of Virology, Dec 29, 2015, 90(6):3253-3256 DOI: 10.1128/JVI.02582-15 PMID: 26719272 PMCID: PMC4810638 NIAID NIH HHS (2)? Grant ID: R01 / AI110964 19 publications Grant ID: R01AI110964 3 publications Google search of Grant ID: R01AI110964 takes us to NIH: National Institute of Allergy and Infectious Diseases (Dr. FAUCI)
DECEMBER 29, 2015 "We report the isolation and characterization of a novel bat coronavirus which is much closer to the severe acute respiratory syndrome coronavirus (SARS-CoV) in genomic sequence than others previously reported, particularly in its S gene. Cell entry and susceptibility studies indicated that this virus can use ACE2 as a receptor and infect animal and human cell lines. Our results provide further evidence of the bat origin of the SARS-CoV and highlight the likelihood of future bat coronavirus emergence in humans." We recently isolated a bat SL-CoV-1 strain (WIV1) and constructed an infectious clone of another strain SHC014 significantly, these strains are closely related to SARS-CoV and capable of using the same cellular receptor (angiotensin-converting enzyme 2 [ACE2]) as SARS-CoV-1. Despite the high similarity in genomic sequences and receptor usage of these two strains, there is still some difference between the N-terminal domains of the S proteins of SARS-CoV-1 and other SL-CoVs, indicating that other unknown SL-CoV-1s are circulating in bats." It was proposed that the S gene from bat-derived CoV, unlike that from human patients- or civets-derived viruses, was unable to use human ACE2 as a receptor for entry into human cells. Civets were proposed to be an intermediate host of the bat-CoVs, capable of spreading SARS CoV to humans. However, in 2013 several novel bat coronaviruses were isolated from Chinese horseshoe bats and the bat SARS-like or SL-SHC014-MA15 was able to use ACE2 from humans, civets and Chinese horseshoe bats for entry. Combined with evolutionary evidence that the bat ACE2 gene has been positively selected at the same contact sites as the human ACE2 gene for interacting with SARS CoV, it was proposed that an intermediate host may not be necessary and that some bat SL-CoVs may be able to directly infect human hosts. To directly address this possibility, the exact S gene from bat coronavirus SL-SHC014 was synthesized and used to generate a chimeric virus in the mouse adapted MA15 SARS-CoV backbone. The resultant SL-SHC014-MA15 virus could indeed efficiently use human ACE2 and replicate in primary human airway cells to similar titres as epidemic strains of SARS-CoV. While SL-SHC014-MA15 can replicate efficiently in young and aged mouse lungs, infection was attenuated, and less virus antigen was present in the airway epithelium as compared to SARS MA15, which causes lethal outcomes in aged mice.
The emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS)-CoV underscores the threat of cross-species transmission events leading to outbreaks in humans. Here we examine the disease potential of a SARS-like virus, SHC014-CoV, which is currently circulating in Chinese horseshoe bat populations. Using the SARS-CoV reverse genetics system, we generated and characterized a chimeric virus expressing the spike of bat coronavirus SHC014 in a mouse-adapted SARS-CoV backbone. The results indicate that group 2b viruses encoding the SHC014 spike in a wild-type backbone can efficiently use multiple orthologs of the SARS receptor human angiotensin converting enzyme II (ACE2), replicate efficiently in primary human airway cells and achieve in vitro titers equivalent to epidemic strains of SARS-CoV. Additionally, in vivo experiments demonstrate replication of the chimeric virus in mouse lung with notable pathogenesis. Evaluation of available SARS-based immune-therapeutic and prophylactic modalities revealed poor efficacy; both monoclonal antibody and vaccine approaches failed to neutralize and protect from infection with CoVs using the novel spike protein. On the basis of these findings, we synthetically re-derived an infectious full-length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo. Our work suggests a potential risk of SARS-CoV re-emergence from viruses currently circulating in bat populations.
While offering preparation against future emerging viruses, this approach must be considered in the context of the US government-mandated pause on GoF studies. Based on previous models of emergence the creation of chimeric viruses like SHC014-MA15 was not expected to increase pathogenicity. However, while SHC014-MA15 is attenuated relative to parental mouse adapted, equivalent studies examining the wild-type Urbani spike within the MA15 backbone produced no weight loss and replication attenuation. As such, relative to the Urbani Spike-MA15 CoV, SHC014-MA15 constitutes a gain in pathogenesis. Based on these findings, review panels may deem similar studies too risky to pursue as increased pathogenicity in mammalian models cannot be excluded. Coupled with restrictions on mouse adapted strains and monoclonal antibodies generated against escape mutants, research into CoV emergence and therapeutic efficacy may be severely limited moving forward. Together, these data and restrictions represent a crossroads of GoF research concerns; the potential to prepare and mitigate future outbreaks must be weighed against the risk of creating more dangerous pathogens. In developing policies moving forward, it is important to consider the value of the data generated by these studies and if they warrant further study or the inherent risks involved.
Reported studies were initiated after the University of North Carolina Institutional Biosafety Committee approved the experimental protocol: Project Title: Generating infectious clones of Bat SARS-like CoVs; Lab Safety Plan ID: 20145741; Schedule G ID: 12279. These studies were initiated prior to the U.S. Government Deliberative Process Research Funding Pause on Selected Gain of Function Research Involving Influenza, MERS, and SARS Viruses and the current manuscript has been reviewed by the funding agency, the National Institutes of Health (NIH). Continuation of these studies have been requested and approved by NIH.
"Editors’ note, March 2020: We are aware that this story is being used as the basis for unverified theories that the novel coronavirus causing SARS-CoV-2 was engineered. There is no evidence that this is true; scientists believe that an animal is the most likely source of the coronavirus. November 20, 2015 In the version of this article initially published online, the authors omitted to acknowledge a funding source, USAID-EPT-PREDICT funding from EcoHealth Alliance, to Z.-L.S. [SHI ZHENG LI] The error has been corrected for the print, PDF and HTML versions of this article." "Declan Butler Senior Reporter, Nature Paris has a degree in biology from Queen's University, Belfast, and a PhD from the University of Leeds. He was made a Chevalier of France's National Order of Merit in 2003 for service to science and society. He wrote: November 12, 2015 An experiment that created a hybrid version of a bat coronavirus — one related to the virus that causes SARS has triggered renewed debate over whether engineering lab variants of viruses with possible pandemic potential is worth the risks. In an article published in Nature Medicine on November 9, 2015, scientists investigated a virus called SHC014 which is found in horseshoe bats in China. The researchers at the University of North Carolina created a chimaeric virus, made up of a surface protein of SHC014 and the backbone of a SARS virus that had been adapted to grow in mice and to mimic human disease. The chimaera infected human airway cells — proving that the surface protein of SHC014 has the necessary structure to bind to a key receptor on the cells ACE2 and to infect them. It also caused disease in mice, but did not kill them. Although almost all coronaviruses isolated from bats have not been able to bind to ACE2, SHC014 is not the first that can do so. In 2013, researchers reported this ability for the first time in a different coronavirus isolated from the same bat population. The findings reinforce suspicions that bat coronaviruses capable of directly infecting humans (rather than first needing to evolve in an intermediate animal host) may be more common than previously thought, the researchers say. [The only corona virus that uses ACE2is Covid-19.]. "The argument is essentially a rerun of the debate over whether to allow lab research that increases the virulence, ease of spread or host range of dangerous pathogens — what is known as ‘gain-of-function’ research. In October 2014, the US government imposed a moratorium on federal funding of such research on the virus that causes SARS. The latest study was already under way before the US moratorium began, and the US National Institutes of Health (NIH) allowed it to proceed while it was under review by the agency, says Ralph Baric, an infectious-disease researcher at the University of North Carolina at Chapel Hill, a co-author of the study. The NIH eventually concluded that the work was not so risky as to fall under the moratorium, Butler says. FULL STORY Researchers from the University of North Carolina at Chapel Hill have discovered a new bat SARS-like virus that can jump directly from its bat hosts to humans without mutation. However, researchers point out that if the SARS-like virus did jump, it is still unclear whether it could spread from human to human. The discovery, reported in the Nov. 9 2015 issue of Nature Medicine, is notable not only because there is no treatment for this newly discovered virus, but also because it highlights an ongoing debate over the government's decision to suspend all gain of function experiments on a variety of select agents earlier this year. The move has put a substantial standstill on the development of vaccines or treatments for these pathogens should there be an outbreak.
"Studies have predicted the existence of nearly 5,000 coronaviruses in bat populations and some of these have the potential to emerge as human pathogens," said senior author Ralph Baric, a faculty member at the Gillings School of Global Public Health and expert in coronaviruses. "So this is not a situation of 'if' there will be an outbreak of one of these coronaviruses but rather when and how prepared we'll be to address it." SARS first jumped from animals to humans in 2002-2003 and caused a worldwide outbreak, resulting in 8,000 cases, including one case in Chapel Hill. With nearly 800 deaths during that outbreak, SARS-CoV presents much like flu symptoms but then can accelerate, compromise breathing and bring on a deadly form of pneumonia. The outbreak was controlled through public health interventions and the original virus was thought to have been extinct since 2004. Baric and his team demonstrated that the newly-identified SARS-like virus, labeled SHC014-CoV and found in the Chinese horseshoe bats, can jump between bats and humans by showing that the virus can latch onto and use the same human and bat receptor for entry. [ACE2] The virus also replicates as well as SARS-CoV in primary human lung cells, the preferred target for infection. "This virus is highly pathogenic and treatments developed against the original SARS virus in 2002 and the ZMapp drugs used to fight Ebola fail to neutralize and control this particular virus," said Baric. "So building resources, rather than limiting them, to both examine animal populations for new threats and develop therapeutics is key for limiting future outbreaks." Journal Reference: Vineet D Menachery, Boyd L Yount, Kari Debbink, Sudhakar Agnihothram, Lisa E Gralinski, Jessica A Plante, Rachel L Graham, Trevor Scobey, Xing-Yi Ge, Eric F Donaldson, Scott H Randell, Antonio Lanzavecchia, Wayne A Marasco, SHI ZHENGLI-LI Ralph S Baric. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature Medicine, 2015; DOI: 10.1038/nm.3985 Simon Wain-Hobson, a virologist at the Pasteur Institute in Paris, points out that the researchers have created a novel virus that “grows remarkably well” in human cells. “If the virus escaped, nobody could predict the trajectory,” he says. Wain-Hobson disapproves of the study because, he says, it provides little benefit, and reveals little about the risk that the wild SHC014 virus in bats poses to humans."The only impact of this work is the creation, in a lab, of a new, non-natural risk,” agrees RICHARD EBRIGHT, a molecular biologist and biodefence expert at Rutgers University in Piscataway, New Jersey. Both Ebright and Wain-Hobson are long-standing critics of gain-of-function research. In their paper, the study authors also concede that funders may think twice about allowing such experiments in the future. "Scientific review panels may deem similar studies building chimeric viruses based on circulating strains too risky to pursue," they write, adding that discussion is needed as to "whether these types of chimeric virus studies warrant further investigation versus the inherent risks involved”. Many other virologists question whether the information gleaned from the experiment justifies the potential risk. Ebright reports he observed no evidence of cutting and pasting genes by humans in SARS-CoV-2. However, "Labs around the globe have been creating synthetic viruses like SARS-CoV-2 for years and its genome would not necessarily contain hallmarks of human manipulation: modern genetic engineering tools permit cutting and pasting genomic fragments without leaving a trace. It can be done quickly, too: it took a Swiss team less than a month to create a synthetic clone of SARS-CoV-2." The coronavirus can warp the body’s defenses in many ways — disarming the body’s early warning systems, for example, or causing immune cells to misfire. But a spate of new studies suggests another insidious consequence: The infection can trigger the production of antibodies that mistakenly attack the patient’s own tissues instead of the virus. he latest report, published online this week, suggests that so-called autoantibodies can persist months after the infection has resolved, perhaps causing irreparable harm. If other studies confirm the finding, it may explain some of the lingering symptoms in people who have recovered from Covid-19. The syndrome, sometimes referred to as long Covid, can include dementia, “brain fog” and joint pain. NYTF
3/7/2021, SARS-CoV-2 has infected over 117,262,992 people and caused more than 2,602,483 deaths. These numbers dwarf the impact of the related SARS coronavirus (SARS-CoV), which caused about 8,000 infections and 800 deaths. Compared to SARS-CoV, many SARS-CoV-2 patients develop low levels of neutralizing antibodies and suffer prolonged illness. SARS-CoV-2 evades the human immune surveillance more effectively than SARS-CoV does. When viruses evolve to escape immune surveillance, they often suffer reduced fitness and become less infectious. Yet SARS-CoV-2 remains highly infectious. In general, SARS-CoV-1 begins with a high fever (temperature greater than 100.4°F [>38.0°C]). Other symptoms may include headache, an overall feeling of discomfort, and body aches. Some people also have mild respiratory symptoms at the outset. About 10 percent to 20 percent of patients have diarrhea. After 2 to 7 days, SARS-CoV-1 patients may develop a dry cough. Most patients develop pneumonia. But sometimes the virus infects people with weak immune systems. In their bodies, the virus can thrive for months. Case studies on these immunocompromised people have shown that the virus can accumulate a large number of mutations as it replicates in their bodies for a long period of time. NYT A small number of Covid patients who had never experienced mental health problems are developing severe psychotic symptoms weeks after contracting the coronavirus. Sporadic cases of post-infectious psychosis and mania have occurred with other viruses, including the 1918 flu and the coronaviruses SARS and MERS.
NYT
Scientists know little about how the virus causes persistent anosmia or how to cure it. But cases are piling up as the coronavirus sweeps across the world, and some experts fear that the pandemic may
leave huge numbers of people with a permanent loss of smell and taste.
NYT
One indication that this virus was enhanced by SHI ZHENG LI is that is doesn't act like any other virus did. It is far more deadly and causes many other symptons other than respiratory disease. It attacks children with a Kawasaki disease - an illness that causes inflammation (swelling and redness) in blood vessels throughout the body. It happens in three phases, and a lasting fever usually is the first sign. The condition most often affects kids younger than 5 years old. Lynne Turner-Stokes, professor of rehabilitation medicine at King’s College, says SARS-CoV-2 is a “multi-system disease” which can potentially affect any organ. It causes microvascular problems and clots. Lungs, brain, skin, kidneys and the nervous system may be affected. Neurological symptoms can be mild (headache) or severe (confusion, delirium, coma). Turner-Stokes says it’s uncertain why the illness is sometimes so protracted. One explanation is that the body’s immune system goes into overdrive, with an ongoing reaction. Another is that the symptoms are virus-driven. Either way, she says there can be a “recrudescence of symptomatology”. Or, as she also puts it using more colloquial language, “the whole caboodle comes back”.
For many doctors, the strange symptomology of long Covid calls to mind another mysterious poorly understood condition: myalgic encephalomyelitis, more familiarly known as chronic fatigue syndrome. ME/CFS, as it is often abbreviated, is defined by the presence of certain symptoms, including debilitating fatigue and unrefreshing sleep, that last for six months or longer. ME/CFS-like syndromes have been linked with infections for more than a century — including, most recently, those caused by the viruses responsible for the SARS and H1N1 pandemics in 2003 and 2009. Chiefly because of this association, several ME/CFS experts told me that they anticipate a wave of new patients — long-haulers who, because their symptoms are severe enough and last for six months or longer, will essentially be ME/CFS patients whether they receive the diagnosis or not. In recent years, scientists have come to realize that the symptoms of certain autoimmune diseases can even mimic psychiatric disorders. In anti-NMDA receptor encephalitis, for example, the immune system attacks glutamate receptors on neurons in the brain, sometimes provoking behavior that resembles what’s seen in schizophrenia. It, too, can be triggered by viral infection. (It’s treatable.) There’s also a pediatric condition that is similar to obsessive-compulsive disorder called pediatric acute-onset neuropsychiatric syndrome, or PANS.
Thread There is a bidirectional relationship between SARS-CoV-2 and diabetes. On the one hand, diabetes is associated with an increased risk of severe SARS-CoV-2. On the other hand, new-onset diabetes and severe metabolic complications of preexisting diabetes, including diabetic ketoacidosis and hyperosmolarity for which exceptionally high doses of insulin are warranted, have been observed in patients with SARS-CoV-2. These manifestations of diabetes pose challenges in clinical management and suggest a complex pathophysiology of SARS-CoV-2–related diabetes. NEJM Although the respiratory system complications of coronavirus disease 19 (COVID-19) have been the most frequent and life threatening, there are increasing reports of central and peripheral nervous system (PNS) involvement. These neurological complications have included encephalopathy, meningo-encephalitis, ischaemic stroke, acute necrotizing encephalopathy, and Guillain-Barré Syndrome (GBS). Radiological series have shown infarcts, microhaemorrhages, features of posterior reversible encephalopathy syndrome, or nerve root enhancement. Zanin and colleagues (2020) have described a case of CNS demyelination post-COVID-19.
The coronavirus can warp the body’s defenses in many ways — disarming the body’s early warning systems, for example, or causing immune cells to misfire. But a spate of new studies suggests another insidious consequence: The infection can trigger the production of antibodies that mistakenly attack the patient’s own tissues instead of the virus. he latest report, published online this week, suggests that so-called autoantibodies can persist months after the infection has resolved, perhaps causing irreparable harm. If other studies confirm the finding, it may explain some of the lingering symptoms in people who have recovered from Covid-19. The syndrome, sometimes referred to as long Covid, can include dementia, “brain fog” and joint pain. https://www.nytimes.com/2021/01/28/health/coronavirus-antibodies-immunity.html
Ron Fouchier, who created an Avian Flu virus that could infect humans, "The question is whether benefits of such research outweigh risks. The answer is not simple. A highly pathogenic bird flu virus transmissible in humans could arise in ways not predicted by laboratory studies. And it is not clear whether this laboratory virus would behave in humans as it does in ferrets. Nonetheless, new data provide valuable insights that can inform influenza preparedness and help delineate the principles of virus transmission between species. Given these uncertainties, important information and insights can come from generating a potentially dangerous virus in the laboratory. While the World Health Organization and the Centers for Disease Control (CDC) and Prevention provide excellent public health surveillance for novel influenza strains, influenza outbreaks still occur suddenly and in unexpected places. The recent H1N1 pandemic exemplifies the problem: In 2009, a new influenza virus emerged. It was shown to have originated from an animal reservoir, and it spread so rapidly that it strained the pharmaceutical industry’s capacity to prepare vaccines fast enough to blunt its spread. We do not fully understand the underlying factors that allow influenza viruses to be transmitted efficiently in humans after they emerge from different species. The ferret transmission studies were intended in part to fill these important gaps in knowledge. Understanding the biology of influenza virus transmission has implications for outbreak prediction, prevention and treatment. In defining the mutations required for mammalian transmission, public health officials are provided with genetic signatures that, like fingerprints, could help scientists more readily identify newly emergent, potentially harmful viruses, track their spread and detect threatening outbreaks. The ability to identify such viruses even a few months faster than by conventional surveillance provides critical time to slow or stop an outbreak. For example, the CDC implements public health protective measures and stockpiles antiviral drugs. Identifying threatening viruses can also facilitate the early stages of manufacturing vaccines that protect against such a virus in advance of an outbreak. In addition, determining the molecular Achilles’ heel of these viruses can allow scientists to identify novel antiviral drug targets that could be used to prevent infection in those at risk or to better treat those who become infected. Decades of experience tells us that disseminating information gained through biomedical research to legitimate scientists and health officials provides a critical foundation for generating appropriate countermeasures and, ultimately, protecting the public health." "One of the goals of pandemic influenza research is to recognize and anticipate how viruses are evolving in the wild toward a phenotype that is dangerous to humans, thereby staying one step ahead of potential pandemics. In this regard, compelling research questions relevant to global health and pandemic preparedness include determining whether highly pathogenic viruses, such as H5N1 have the ability to mutate and/or reassort with another influenza virus to become readily transmissible by the airborne route among humans. If so,
"Within this context, global attention has been paid recently to two NIH-funded studies of H5N1 transmissibility and pathogenesis in ferrets. In those studies, H5N1 viruses were made transmissible via respiratory droplets among ferrets by engineering the virus; well-described and published protocols including reverse genetics, reassortment, and passaging of viruses in mammals were used. Manuscripts describing the studies have generated an unprecedented degree of discussion, concern, and disagreement among scientists, as well as the public, regarding whether the experiments should have been performed in the first place and whether they should be published in their entirety. Major sources of concern have been that the results might be used by bioterrorists to harm the public or that the virus might accidentally escape and cause a pandemic."
"However, whenever one deliberately manipulates a virus or a microbe, it is always possible, at least theoretically, that the research results could be used by bioterrorists to intentionally cause harm, or that an accidental release of a pathogen from a laboratory could inadvertently cause harm. Such research is referred to as “dual-use research,” as the research potentially has both positive and negative applications. A particular subset of dual-use research is referred to as “dual-use research of concern” or DURC. DURC is defined as life sciences research that, on the basis of current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that can be directly misapplied to pose a significant threat with broad potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security. If a particular experiment is identified as DURC, that designation does not inherently mean that such research should be prohibited or not widely published. However, it does call for us to balance carefully the benefit of the research to public health, the biosafety and biosecurity conditions under which the research is conducted, and the potential risk that the knowledge gained from such research may fall into the hands of individuals with ill intent. Research that could enhance the transmissibility of H5N1 viruses clearly is DURC."
"In this regard, the question of whether to publish the two H5N1 studies in ferrets has been intensively discussed by an independent federal advisory committee known as the National Science Advisory Board for Biosecurity (NSABB). On the basis of their recommendations and other evaluations, the U.S. government agreed that the research is important for the public health and should be published. However, important lessons were learned along the way and, appropriately, triggered an examination of our approach concerning the conduct, oversight, and communication of DURC. In this regard, the U.S. government announced on March 29, 2012 the U.S. Government Policy for Oversight of Life Sciences Dual Use Research of Concern. This policy document outlines, for federal departments and agencies that conduct or fund life sciences research, steps to determine whether projects fall under the definition of DURC to assess the risks and benefits of these projects, to review them regularly, and to develop risk mitigation plans. In the process of weighing the potential risks and benefits of publishing these two manuscripts, it also became clear that, when possible, it is critical to identify research with DURC potential before the initiation of the project and, certainly, before the results are submitted for publication. Such monitoring in the case of NIH-funded research requires the concerted effort of all involved, including scientists applying for or in receipt of NIH funding and NIH program officials. Additional guidelines will be needed as well to assist biosafety committees in evaluating DURC at the institutions where the research is conducted. Benefits and Risks of Influenza Research: Lessons Learned ANTHONY S. FAUCI, Francis S. Collins Science 22 Jun 2012: Vol. 336, Issue 6088, pp. 1522-1523 DOI: 10.1126/science.1224305." "The Education Department has asked the University of Texas System to provide documentation of its dealings with the Chinese laboratory U.S. officials are investigating as a potential source of the coronavirus pandemic. The request for records of gifts or contracts from the Wuhan Institute of Virology and its researcher SHI ZHENG LI known for her work on bats, is part of a broader department investigation into possible faulty financial disclosures of foreign money by the Texas group of universities. As one of the nation's 14 Bio Safety Level 4 laboratories, the Galveston National Laboratory at UTMB belongs to the National Institute of Allergy and Infectious Diseases (NIAID) Biodefense Laboratory Network. It has collaborated with more than 70 countries and with scientists from the U.S. and abroad on biosafety and biosecurity, as part of its broad mission to advance global scientific collaboration. While it receives no financial support or gifts from global scientific laboratories, UTMB complies with obligations to report fiduciary relationships as required by statute." O'Keeffe, Wall Street Journal 5/1/2020 It cuts against the deep public faith in Fauci, but White House officials have long pointed to the fact that he didn't favor travel bans early on and downplayed the idea of person-to-person transmission https://t.co/19GkhEh2a4 "The Office of the Director (OD) determines Institute programs, plans, and policies and provides management, program analysis, and scientific program reporting services to the Institute, as well as scientific leadership, policy guidance, and overall operational and administrative coordination. The OD serves as the chief liaison with the National Institutes of Health (NIH) director, other components of of the U.S. Department of Health and Human Services (HHS), other federal agencies, Congress, professional societies, voluntary health organizations, and other public health groups. It also coordinates the activities of NIAID extramural and intramural divisions. "GoF studies, or research that improves the ability of a pathogen to cause disease,
help define the fundamental nature of human-pathogen interactions, thereby enabling assessment of the pandemic potential of emerging infectious agents, informing public health and
preparedness efforts, and furthering medical countermeasure development. Gain-of-function studies may entail biosafety and biosecurity risks; therefore, the risks and benefits of gain-offunction research must be evaluated, both in the context of recent U.S. biosafety incidents and to keep pace with new technological developments, in order to determine which types of studies
should go forward and under what conditions. In light of recent concerns regarding biosafety and biosecurity, effective immediately, the U.S.
Government (USG) will pause new USG funding for gain-of-function research on influenza, MERS or SARS viruses, as defined below. This research funding pause will be effective until a
robust and broad deliberative process is completed that results in the adoption of a new USG gain-of-function research policy. Restrictions on new funding will apply as follows: New USG funding will not be released for gain-of-function research projects that may be reasonably anticipated to confer attributes to influenza, MERS, or SARS viruses such that the virus would have enhanced pathogenicity and/or transmissibility in mammals via the respiratory route. The research funding pause would not apply to characterization or testing of naturally occurring influenza, MERS, and SARS viruses, unless the tests are reasonably anticipated to increase transmissibility and/or pathogenicity."
"As this report was being finalized, CDC leadership was made aware that earlier this year a culture of low-pathogenic avian influenza was unintentionally cross-contaminated at a CDC influenza laboratory with a highly pathogenic H5N1 strain of influenza and shipped to a BSL-3, select-agent laboratory operated by the United States Department of Agriculture (USDA). The CDC influenza laboratory where this incident occurred is now closed and will not reopen until adequate improvements are put in place. Although CDC is continuing to investigate and review this matter, Attachment A provides current information on the incident and the agency's response." [COMMENT: Most of these accidents involved the shipment of live anthrax bacteria initially believed to be inactivated exposed lab technicians to contamination.] The SARS 2004 outbreak in China, with an initial eight confirmed or suspected cases and hundreds quarantined, involved two researchers who were working with the virus. Both worked at the Chinese Institute of Virology in Beijing, part of China's Center for Disease Control. In the early 2000s, there were three documented cases of the original SARS virus escaping from a laboratory environment, according to Lim Poh Lian, a senior consultant at the National Centre for Infectious Diseases in Singapore. In the case of a 2003 incident in Singapore, a student became infected with SARS after his samples of West Nile virus were cross-contaminated with live SARS virus. That virus was being grown in relatively large quantities in the lab for studies on the disease, and the student was not properly trained in safety procedures for the lab he was working in. Incidents causing potential exposures to pathogens occur frequently in the high security laboratories often known by their acronyms, BSL3 (Biosafety Level 3) and BSL4. Lab incidents that lead to undetected or unreported laboratory-acquired infections can lead to the release of a disease into the community outside the lab; lab workers with such infections will leave work carrying the pathogen with them. If the agent involved were a potential pandemic pathogen, such a community release could lead to a worldwide pandemic with many fatalities. Of greatest concern is a release of a lab-created, mammalian-airborne-transmissible, highly pathogenic avian influenza virus, such as the airborne-transmissible H5N1 viruses created in the laboratories of Ron Fouchier in the Netherlands and Yoshihiro Kawaoka In Madison Wisconsin. In an analysis circulated at the 2017 meeting for the Biological Weapons Convention, a conservative estimate shows that the probability is about 20 percent for a release of a mammalian-airborne-transmissible, highly pathogenic avian influenza virus into the community from at least one of 10 labs over a 10-year period of developing and researching this type of pathogen. This percentage was calculated from Federal Select Agent Program incident data. FSAP incident data were collected from summary reports to Congress for the years 2004 through 2010. [COMMENT: This is in the USA with a much lower accident rate than China.]
"The US National Institutes of Health (NIH) funded bat-coronavirus research in the Wuhan Institute of Virology in China to the tune of US$3.7 million. Back in October 2014, the US government had placed a federal moratorium on gain-of-function (GOF) research – altering natural pathogens to make them more deadly and infectious – as a result of rising fears about a possible pandemic caused by an accidental or deliberate release of these genetically engineered monster germs." The funding went to an intermediate organization known as ECOHEALTH ALLIANCE run by Peter Daszak. A portion of $3.7 million in grants awarded between 2014 and 2019 by the National Institutes of Health (NIH) to EcoHealth Alliance, a global environmental health nonprofit organization, helped fund research at the Wuhan Institute of Virology in China. However, not all of that $3.7 million went to the Wuhan Institute of Virology, and not all of the funding took place under the Obama administration. Approximately $700,000 of the $3.7 million total was approved under Donald "Clorox" Trump who suggested household cleaning agents should be ingested to fight SARS-CoV-19.
ONE OF MANY MISSTEPS
This virus did not originate in the Huanan South China Seafood Market as first believed. No SARS-CoV-2 isolates were detected in any of the animals or fish sold at the market, only in environmental samples, including sewage. SARS-CoV-2 originated "5 or 6 bus stops away" with an invalid who never went to the wet market. Many China scholars noted that it was quite unusual for Chinese government authorities to identify Wuhan’s Huanan South China Seafood Market so quickly as the source of the outbreak. They thought this behavior so uncharacteristic that it raised suspicions in their minds. The authors also noted that “[n]o animal sampling prior to the shutdown and sanitization [of the Wuhan fish market] was done.”
Shing Hei Zhan, Benjamin E. Deverman and Yujia Alina Chan, “SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence?” BioRxiv, posted May 2, 2020 on: https://doi.org
Early investigations into SARS-CoV-2 emergence and the resulting SARS-CoV-2 disease outbreak proposed the proximate cause was a zoonotic spillover event in late November or early
December 2019 in Wuhan, China. This was supported by preliminary epidemiological studies, including the initial clinical series which linked two-thirds of the identified cases to the Huanan
Seafood Market in Wuhan. Critically, the study found no direct connection to the market for 14 individuals, including the first known case of SARS-CoV-2, leaving open the possibility of alternate
points of origin and infection. Additionally, virologic samples of wildlife in the Huanan market could not be linked to SARS-CoV-2, suggesting transmission at the market was downstream from the spillover event. Here we consider that SARS-CoV-2 may have already been circulating in the community prior to the identification of the Huanan Market cluster. This hypothesis is supported by emerging epidemiologic and phylogenetic evidence indicating that the virus emerged in southern China and may have already spread internationally, and
adapted for efficient human transmission by the time it was detected in late December. "The new virus revealed itself gradually in humans last December 2019, in Wuhan, and in January 2020 several Chinese laboratories, including SHI ZHENG LI - a Chinese virologist who researches SARS-like coronaviruses of bat origin. SHI ZHENG LI directs the Center for Emerging Infectious Diseases at the Wuhan Institute of Virology, WIV a biosafety level 4 laboratory located in Jiangxia District, Wuhan. Her lab sequenced wholly or partly the genomes of samples from different patients, including five complete genomes. SHI ZHENG LI and her colleagues made their announcement on January 23, 2020 that the virus found in those five patients was 96.2-per-cent identical to the bat coronavirus they had warned about three years earlier." [As stated the 4% differences is thousands of genomes. The viruses were SIMILAR not identical and the virus was not an evolutionary cousin of CoV-19 nor could it cause disease in humans]. "By that time, the virus had been circulating in Wuhan for at least seven weeks, and three consequential misconceptions had been propagated, not just by political leaders but by hospital officials and the Chinese version of the C.D.C.:
"On the second and third points, there was disagreement by clinicians treating patients, such as Zhang Li, at Wuhan’s Jinyintan Hospital, who told the Wall Street Journal in late February, “I was on alert because this was a new pneumonia and because I’d dealt with SARS.” The misconception that the market was the origin of the outbreak is implicitly contradicted by a scientific paper published in late January 2020, by a group of physicians from Wuhan and Beijing, describing clinical features of the first forty-one patients. Twenty-seven of them, the paper said, had been exposed in the Huanan market. Since a single horseshoe bat would be unlikely to infect twenty-seven people, even if they diced it into hors d’œuvres, and since by some accounts bats weren’t even on sale in the market at that time, a few scientists have speculated that there was an intermediate host animal—a snake, a pangolin, a palm civet?—in which the virus amplified itself, before that larger creature was sold or butchered. The molecular evidence for snakes is weak, pangolins are a complicated story, and civets aren’t implicated this time." Speculations that pangolins are the likely intermediate animal host stemmed from the discovery of a pangolin CoV that shares 95.4% S amino acid identity and six key receptor-binding domain residues with SARS-CoV-2. Since then, another closely related lineage of pangolin CoVs has been identified. However, the unique polybasic furin cleavage site PRRA in the SARS-CoV-2 S is not found in pangolin CoVs (42), and SARS-CoV-2 is not a recent recombinant involving any of the CoVs sampled to date. As previouosly stated the CoV that is most closely related to SARS-CoV-2 is RaTG13 a bat CoV that was identified at the Wuhan Institute of Virology and originally isolated from the Yunnan Province of China. RaTG13 shares 96.2% genome identity with the Wuhan-Hu-1 SARS-CoV-2 isolate. In comparison, the most closely related pangolin CoV MP789 shares only 84.1% and 84.0% genome identity with Wuhan-Hu-1 and RaTG13, respectively. No evidence as yet points to the adaptation of SARS-CoV-2 for human infection in pangolins or the transmission of SARS-CoV-2 from pangolins to humans. The genome-wide phylogenetic tree indicated that SARS-CoV-2 was closest to RaTG13, followed by GD Pangolin SARSr-CoV, then by GX Pangolin SARSr-CoVs, then by ZC45 and ZXC21, then by human SARS-CoV, and finally by BM48-31(Fig. 1A). Notably, we found that the nucleotide divergence at synonymous sites between SARS-CoV-2 and other viruses was much higher than previously anticipated. For example, although the overall genomic nucleotides differ ~4% between SARS-CoV-2 and RaTG13, the genomic average dS was 0.17, which means the divergence at the neutral sites is 17% between these two viruses (Table 1). Note that nonsynonymous sites are usually under stronger negative selection than synonymous sites, and calculating sequence differences without separating these two classes of sites may underestimate the extent of molecular divergence by several folds. Coronaviruses are naturally hosted and evolutionarily shaped by bats. Indeed, it has been postulated that most of the coronaviruses in humans are derived from the bat reservoir. Several teams have recently confirmed the genetic similarity between SARS-CoV-2 and a bat betacoronavirus of the sub-genus Sarbecovirus (the viral subgenus containing SARS-CoV and SARS). The whole-genome sequence of the novel virus has 96.2% similarity to that of a bat SARS-related coronavirus (SARSr-CoV; RaTG13) collected in Yunnan province, China, but has low similarity to that of SARS-CoV (about 79%) or MERS-CoV (about 50%). It has also been confirmed that the SARS-CoV-2 uses the same receptor, the Angiotensin Converting Enzyme II (ACE2), as the SARS-CoV. Although the specific route of transmission from natural reservoirs to humans remains unclear several studies have shown that pangolins may have provided a partial spike gene to SARS-CoV-2; the critical functional sites in the spike protein of SARS-CoV-2 are nearly identical to those identified in a virus isolated from a pangolin. Despite these recent discoveries, several fundamental issues related to the evolutionary patterns and driving forces behind this outbreak of SARS-CoV-2 remain to be fully characterized.Both SARS-CoV and SARS-CoV-2 bind to ACE2through the RBD of the spike protein in order to initiate membrane fusion and enter human cells. Five out of the six critical amino acid (AA) residues in RBD were different between SARS-CoV-2 and SARS-CoV, and a 3D structural analysis indicated that the spike of SARS-CoV-2 had a higher binding affinity to ACE2than SARS-CoV. Intriguingly, these same six critical AAs are identical between GD Pangolin-CoV and SARS-CoV-2. In contrast, although the genomes of SARS-CoV-2 and RaTG13 are more similar overall, only one out of the six functional sites are identical between the two viruses. It has been proposed that the SARS-CoV-2 RBD region of the spike protein might have resulted from recent recombination events in pangolins. Although several ancient recombination events have been described in spike it also seems likely that the identical functional sites in SARS-CoV-2 and GD Pangolin-CoV may actually result from coincidental convergent evolution. "Coincidental convergent evolution" one thing had nothing to do with the other.
The genome-wide phylogenetic tree indicated that SARS-CoV-2 was closest to RaTG13 followed by GD Pangolin SARSr-CoV, then by GX Pangolin SARSr-CoVs, then by ZC45 and ZXC21, then by human SARS-CoV, and finally by BM48-31(Fig. 1A). Notably, we found that the nucleotide divergence at synonymous sites between SARS-CoV-2 and other viruses was much higher than previously anticipated. For example, although the overall genomic nucleotides differ ~4% between SARS-CoV-2 and RaTG13 , the genomic average dS was 0.17, which means the divergence at the neutral sites is 17% between these two viruses (Table 1). Note that nonsynonymous sites are usually under stronger negative selection than synonymous sites, and calculating sequence differences without separating these two classes of sites may underestimate the extent of molecular divergence by several folds. "An American physician and scholar Daniel R. Lucey (who had also worked the SARS outbreak, in Guangzhou, Hong Kong, and Toronto) wondered about the 14 other early patients. He noticed that the first of them, falling sick on December 1, 2019 had no direct or secondary contact with the market. That meant, given the incubation period, that the virus must have been circulating in Wuhan outside the market since November 2020. This doesn’t controvert the likelihood of the virus originating in a bat, but it suggests that perhaps it went into the Huanan market, as well as coming out of it, in humans."
Some bats migrate to warmer areas while others hibernate. Once insect activity begins to decline and bats begin looking for a place to hibernate (or enter a state or prolonged torpor). Bats begin hibernating when the cold weather drives the insects away, typically around October and November, and emerge from hibernation in March. Bat hibernation patterns can vary by region, based on seasonal temperature differences across the country. While some bats are capable of activity during their hibernation period, they usually remain inactive due to their unique self-preservation process. When a bat hibernates, its metabolism slows down to conserve energy. Each day its body cycles in and out of a deep resting state known as torpor, in which the bat's heartbeat slows from 200-300 beats per minute to as few as 10 beats per minute. This maintains the bat’s energy level at 2 percent of normal life functions and allows it to survive for up to six months on a very small amount of stored body fat. A bat can lose as much as half of its body weight during hibernation. The torpor state also allows bats to adapt to their surroundings. Bats can lower their body temperature from a normal level of 100 degrees or more all the way to 40 degrees or less as needed to preserve energy. "The logic seems straightforward. But a more complete analysis of early cases suggests that locating the origin of the virus may not be so simple. A study published in the New England Journal of Medicine A separate Jan. 24, 2020 analysis published in the Lancet found that three of the first four cases — including the first known case — did not have market links [and neither did 13 of the first 41 confirmed cases]: "From Jan 10, 2020, we enrolled a family of six patients who travelled to Wuhan from Shenzhen between Dec 29, 2019 and Jan 4, 2020. Of six family members who travelled to Wuhan, five were identified as infected with the novel coronavirus. Additionally, one family member, who did not travel to Wuhan, became infected with the virus after several days of contact with four of the family members. None of the family members had contacts with Wuhan markets or animals, although two had visited a Wuhan hospital. Five family members (aged 36–66 years) presented with fever, upper or lower respiratory tract symptoms, or diarrhoea, or a combination of these 3–6 days after exposure. They presented to our hospital (The University of Hong Kong-Shenzhen Hospital, Shenzhen) 6–10 days after symptom onset. They and one asymptomatic child (aged 10 years) had radiological ground-glass lung opacities."
"Scientific American quotes Dr. Kevin Olival Vice President for Research at EcoHealth Alliance a group that has been implicated in funding the pandemic: "How and where the SARS-CoV-2 spillover occurred is not known for certain. There was an early suspicion that the initial outbreak could have started at the Huanan Seafood Wholesale Market in Wuhan, which was closed on January 1 2020. But “we don’t know if the spillover happened outside the market and then began spreading after it was brought there. It is also unclear if there was an intermediate animal host between the disease-carrying bats and humans." Dr. Olival’s role as Senior Research Scientist at EcoHealth Alliance involves coordinating the modeling and analytics research; integrating evolutionary and ecological theories to understand the drivers of disease emergence; and managing zoonotic disease surveillance efforts in Thailand and Indonesia under the USAID PREDICT project.
In an effort to identify and respond to new zoonotic diseases before they spread to humans, the U.S. Agency for International Development (USAID) established its Emerging Pandemic Threats (EPT) program. The EPT program consists of four projects: PREDICT, RESPOND, IDENTIFY, and PREVENT. The PREDICT project seeks to identify new emerging infectious diseases that could become a threat to human health. PREDICT partners locate their research in geographic "hotspots" and focus on wildlife that are most likely to carry zoonotic diseases - animals such as bats, rodents, and nonhuman primates.
EcoHealth Alliance, a PREDICT partner, created a picture book, Living Safely With Bats, in 12 languages. PREDICT field teams used the book in education campaigns in villages in Sierra Leone and the forest region of Guinea. The message: Don't eat the animals or fruit they may have contaminated. But don't exterminate them either, because they are important pollinators, they protect crops by eating beetles and other pests, and they can spread infection if they are disrupted. National Geographic
Despite referencing Scientific American and NEJM to show the virus didn't start at the market the Washington Post claimed it was doubtful the virus orginated at WIV. Why? "conspiracy theories."
CONCLUSION: The Chinese government’s poor record of transparency; the fact that the Wuhan Institute of Virology, a research center with facilities in the same city where the virus first appeared, was studying and creating new dangerous pathogens, including bat coronaviruses cannot be easily dismissed.
The WSJ reported: "WANG YANYI has ruled out both a laboratory and an animal market in the city of Wuhan as possible origins of the coronavirus pandemic, in their most detailed pushback to date against allegations from U.S. officials and others over what might have sparked it. The director of the Wuhan Institute of Virology, at the center of allegations around a potential laboratory accident, Dr. WANG YANYI, over the weekend told China Central Television that the coronavirus was significantly different from any live pathogen that has been studied at the institute and that there therefore was no chance it could have leaked from there." Editors Note: The RaTG13 which was touted as a close relative to SARS-CoV-2 was stored at the WIV. I’m glad the reporters at @nytimes recognized the incredibly fast work and open sharing of data from our colleagues at Wuhan Institute of Virology led by SHI ZHENG LI and Peng Zhou. https://t.co/F795ou1tXY When peterdaszak.com went online the first ones to visit it within minutes were the Chinese. They had an alert on their agent of influence Peter Daszak. But visiting the page so quickly they confirmed the fact he was a Chinese agent or agent of influence.
PETER DASZAK is not a microbiologist but holds a B.Sc. in Zoology in 1987 at University College of North Wales (UCNW), and a Ph.D. in parasitic infectious diseases in 1994 at University of East London. Peter was a prime target of Chinese intelligence recruitment. He parrots the Party Line. He also worked with the CIA heading PROJECT ECOHEALTH CIA until Trump cut off his funds. PROJECT ECOHEALTH is a think tank put together by the CIA that was supposed to protect America from Pandemics by keeping an eye on bats. But ECOHEALTH began to subsidize Gain-of-Function experiments at WIV and a recombinant virus created by SHI ZHENG LI leaked from the lab into the streets of Wuhan then into the market and out again. Was it coincidence that SARS-CoV-2 started close to WIV? Just how close the Chinese won't say as they refuse to give out the address of patient ZERO.
Dr. Daszak is a member of the National Academy of Medicine and Chair of the NASEM’s Forum on Microbial Threats. He is a member of the NRC Advisory Committee to the US Global Change Research Program, the Supervisory Board of the One Health Platform, the One Health Commission Council of Advisors, the CEEZAD External Advisory Board, the Cosmos Club, and the Advisory Council of the Bridge Collaborative. He has served on the IOM Committee on global surveillance for emerging zoonoses, the NRC committee on the future of veterinary research, the International Standing Advisory Board of the Australian Biosecurity CRC; and has advised the Director for Medical Preparedness Policy on the White House National Security Staff on global health issues. Dr. Daszak is a regular advisor to WHO on pathogen prioritization for R&D. Dr. Daszak won the 2000 CSIRO medal for collaborative research on the discovery of amphibian chytridiomycosis, is the EHA institutional lead for USAID-EPT-PREDICT, is on the Editorial Board of Conservation Biology, One Health, GeoHealth, One Health Outlook, Transactions of the Royal Society of Tropical Medicine & Hygiene, and is Editor-in-Chief of the journal Ecohealth. He has authored over 300 scientific papers and was listed as a Web of Science Highly Cited Researcher in 2018. His work has been the focus of extensive media coverage, ranging from press articles in The New York Times, the Wall Street Journal, The Economist, The Washington Post, US News & World Report, and broadcast appearances on 60 Minutes, CNN, ABC, NPR’s Talk of the Nation, Science Friday, and Fresh Air with Terry Gross. PETER DASZAK would be a prime target of Chinese intelligence recruitment as he is already in bed with the CCP. He parrots the Party Line. But he was co-opted by the CIA until Trump cut his funds off. ECOHEALTH is a think tank put together by the CIA that was supposed to protect America from Pandemics by keeping an eye on bats. But they began to subsidize Gain-of-Function experiments at WIV and a recombinant virus leaked from the lab. Was it coincidence that SARSCov2 started close to WIV? Just how close the Chinese won't say.
PETER DASZAK and SHI ZHENG LI were inducing Gain of Function (GoF) mutations in the SARS CoV-1 virus in order to get one step ahead of evolution by determining what the next viral threat to humanity would be. In collaboration with American Universities, arranged by Daszak, they produced a new Corona virus capable of infecting human beings. Because viruses mutate so readily during their replication, this new virus had to be checked to make sure it only has the mutations the lab caused. But this was overlooked by the virologists and new and even more deadly virus emerged which leaked from the aging biocontainment level 4 lab into Wuhan via a lapse in biosecurity. This might have been the result of an asymptomatic carrier, or improper disposal of bio-hazardous waste? As stated the virus did not originate in the Wuhan Wet Market as first believed.
"Look, first, the idea that this virus escaped from a lab is just pure baloney. It’s simply not true. I’ve been working with that lab for 15 years. And the samples collected were collected by me and others in collaboration with our Chinese colleagues. They’re some of the best scientists in the world. There was no viral isolate in the lab. There was no cultured virus that’s anything related to SARS coronavirus 2. So it’s just not possible.* Now, how did it get into the market? We know for sure that the Wuhan market was part of this outbreak, but we think that the first few cases weren’t in the market. And this is not uncommon. We’ve seen this with many, many other disease outbreaks, new viruses that emerge. They trickle out from rural areas through a person getting infected maybe in Hunan province and then moving into Wuhan, that maybe they’re part of the wildlife trade. Maybe a farmer got infected, or a farmer’s animals, and they were shipped into the markets. These wet markets aren’t just places to sell wildlife; they’re places where people congregate. They come in in droves. They circulate around. They’re really good places for a virus to spread. And if a person brings it in, or an animal, that virus will spread. And it looks like that’s what’s happened here." Dr. WANG YANYI said the institute now had three strains of live viruses, including one that was 96% genomically similar to the SARS virus. But their highest similarity to SARS-CoV-2 was only 79.8 per cent, Dr. WANG YANYI said.
If the disease "trickles out from rural areas" (because there are as many bats in Wuhan as there are in New York City) why didn't someone in those rural areas become infected first? Why wasn't Hunan put under lock-down? "Maybe a farmer got infected, or a farmer’s animals, and they were shipped into the markets." Why did the market closest to the Wuhan Institute of Virology get infected and no other wet markets? As documented at the beginning of this page the virus did not originate in the market but this Chinese agent of influence sticks with a variation of the market story.
"Before accepting the first batch of test samples from SARS-CoV-2 patients on December 30, 2019, the institute’s labs did not have the novel coronavirus and no one at the institute has contracted SARS-CoV-2 to this day. “It is impossible for the virus to leak from such a high-security laboratory,” said Yuan Zhiming, a researcher at the Wuhan Institute of Virology in an interview with CGTN on April 18, 2020. The claim that the novel coronavirus escaped from the laboratory is pure nonsense, said PETER DASZAK , chairperson of EcoHealth Alliance, a New York-based non-profit organization, who has been cooperating with the Wuhan Institute of Virology for 15 years. There is no virus cultivation related to the new coronavirus in the laboratories of the Wuhan Institute of Virology, so the so-called “laboratory leak theory” is impossible, said PETER DASZAK who is responsible for studying emerging infectious diseases worldwide." Simply untrue as evidenced by the scientific papers generated by the Institute. PETER DASZAK on NPR "The real risk is in the wild in the way people interact with wildlife around the world," says PETER DASZAK , president of EcoHealth Alliance. "That's where we need to be focused if we want to really do something about preventing the next pandemic. We're finding 1 to 7 million people exposed to these viruses every year in Southeast Asia; that's the pathway. It's just so obvious to all of us working in the field," he says. No conspiracy theories. Despite the evidence, misinformation about the virus's origins continue to proliferate. For PETER DASZAK who has worked on other outbreaks, the pattern is all too familiar: "Every time we get a new virus emerging, we have people that say, 'This could have come from a lab,' " he says. What's unusual about this outbreak, he says, is that senior officials in both China and the U.S. have traded accusations that each nation is somehow responsible for the virus. "It's a real shame that the conspiracy theories can get to the level they've got with policymakers," he says. The political heat has strained the very scientific collaborations meant to detect these viruses as they emerge, warns Jonna Mazet. The PREDICT system she runs relies on the voluntary cooperation of many countries, with researchers freely sharing data about viruses they're spotting within their borders. "With this sort of blame game that's going on, we put ourselves at much more risk." PETER DASZAK says the time for finger-pointing is over. "We have a bat virus in my neighborhood in New York* killing people," he says. "Let's get real about this." PETER COTTINGTON DASZAK lives in the country at 60 VIOLA RD, SUFFERN, NY 10901-3209
Do you think that we will ever know where SARS-Cov-2 came from – and does it matter?
It does matter, but we're never going to know 100 per cent for sure – it's very hard to definitively prove, without being there at the time. In that space of uncertainty, conspiracy theories abound – for instance the idea it could have been bio-engineered. This is ridiculous, but it’s time consuming to fight these fires. I think it will take a couple of years to track Sar-Cov-2 properly. We need to trace back the sorts of animals that were going into Wuhan’s wet market – and the people. It’s quite possible it was circulating on a farm for weeks or months before emerging in Wuhan. Every time we've looked at the origins of the new disease in the past, we’ve found out it was actually around a lot longer than thought. Perhaps we will find it was just missed because, in most people, it just causes a cough – who in rural China doesn't have a cough every now and again?
What do you think is driving the conspiracy theories we’ve seen over the last few months?
People buy into conspiracy theories because they’re convincing stories told by charismatic people with just enough science and fact to make them seem plausible. The truth also tends to be less interesting. In this case, people in China are exposed to bats, who harbour viruses. It’s a lot more exciting to blame scientists inside a top secret lab that’s blocked off with barbed wire even if what is going on behind those doors is actually pretty boring.
Do you think that our natural propensity to jump on conspiracy theories is being played on by politicians?
Clearly this is part of the politics now. Take the US. We're seeing conspiracy theories being elevated in a polarised press, to the point where politicians then use that to make policy decisions. Which is pretty frightening.
Who are your heroes and villains of the pandemic?
Then there are people like SHI ZHENG LI in China, who has stuck through death threats and a complete disparaging of her character, just to do what she does: try to save lives.
https://www.telegraph.co.uk/global-health/science-and-disease/will-see-outbreaks-inevitable-warning-worlds-famous-virus-hunter
DASZAK is sticking with the Wuhan Market origination myth. "It’s a lot more exciting to blame scientists inside a top secret lab that’s blocked off with barbed wire even if what is going on behind those doors is actually pretty boring." There is only a cyclone fence around WIV and making new virus is not boring. Finally the only cases in rural China where bats abound were characterized by a mild cough or no symptoms. No rural Chinese came down with full blown SARS-Cov-2.
"A grant to a New York nonprofit ECOHEALTH ALLIANCE aimed at detecting and preventing future outbreaks of coronaviruses from bats has been canceled by the National Institutes of Health, Politico reports, apparently at the direction of President Donald Trump because the research involved the Wuhan Institute of Virology in China. On April 14, the The Washington Post published a column highlighting State Department cables about concerns regarding safety at the institute."
This Operation is built around the PREDICT program that failed the American people. The scientists involved in EcoHealth are wildlife / human interface scholars and have no idea of what went on in WIV.
Tom Hughes Senior Scientist As Malaysian Project Coordinator at EcoHealth Alliance, Tom Hughes coordinates all of our sampling, testing, capacity building and training efforts in Malaysia. Tom’s responsibilities include setting up and running the Study of Zoonotic Infections among Persons Exposed to Wild Animals, a collaborative research project with the Malaysian Government, as well as being the PREDICT country coordinator for the USAID Emerging Pandemic Threats program and Deputy Chief of Party for the USAID Infectious Disease Emergence and Economics of Altered landscapes (IDEEAL) Project. Tom began working with EcoHealth Alliance in June 2005 on the Nipah virus research project in Malaysia. In 2007, he took on the new role of coordinating the Study of Zoonotic Infections among Persons Exposed to Wild Animals in Malaysia, to determine if close contact with wild animals results in the transfer of zoonotic diseases. In 2010, Tom became the PREDICT Malaysia country coordinator for USAID’s Emerging Pandemic Threats program. The aim of this research is to create an early warning system for potential zoonotic disease spillover into livestock and humans. Tom is working closely with partners from the Ministry of Health, the Department of Wildlife and National Parks, the Department of Veterinary Services, Sabah Wildlife Department and local universities to develop personnel and laboratory capacity and establish sustainable disease surveillance systems. Dr. William B. Karesh is the Executive Vice President for Health and Policy at EcoHealth Alliance. He serves as the president of the World Animal Health Organization (OIE) Working Group on Wildlife Diseases and also chairs the International Union for the Conservation of Nature (IUCN) Wildlife Health Specialist Group, a network of wildlife and health experts around the world. Dr. Karesh also serves on the World Health Organization's (WHO) International Health Regulations Roster of Experts focused on the human-animal interface and wildlife health. Currently, Dr. Karesh is the EPT Partner Liaison for the USAID Emerging Pandemic Threats PREDICT-2 program, a >$120 million effort focused on predicting and preventing pandemic diseases. He is a Life Member of the Council on Foreign Relations. “Every single outbreak of a novel virus, somebody somewhere says, ‘Well this has been manufactured in a lab.’ … We estimate there are 1.7 million unknown viruses in wildlife, so there’s a lot of diversity out there and we need to be looking at that instead of pointing fingers." https://t.co/OJjvyWulEp A recent Pew study found that one-in-three Americans believes a theory that the virus behind COVID-19 was lab released. There is no science to back this up. https://t.co/IlOO5vFuws Academician Xi Chong Nanshan has already delivered a speech on TV. Wuhan New Coronavirus is not the true source of the virus. There are 5 new coronavirus series, ABCDE bat sleeve BAT (H52) and then mutates H53> The C family pathogens in China, with variants of C56 and MV2, spread throughout the world. But the United States had five major families of ABCDE two years ago, the US Government CDC (Center Disease of Control) Concealing the illness, when a military contest was held in Wuhan in November, two American soldiers went to the Wuhan Hospital in Hubei to catch a cold. It was the first hospital in Wuhan, China, which discovered a coronavirus transmission in early December (Golden Eagle) Tan Hospital and Wuhan Central Hospital). From this calculation, it should be that the US soldiers brought the virus into Wuhan in the Wuhan military competition, and the virus series H1 (referred to as the son) came to China. If China wants to check the origin of these two American blood sources, we can confirm the source. The US should have ABCDE two years ago (H13, H3, H1, H56, MV2) These five kinds of viruses have hundreds of pathogens in bats. Among them, the United States has mutated five kinds of pathogens. The United States has always been a stable fact. This is why the U.S. government is in a hurry to send them Americans back to the United States, and is afraid to find pathogens from Americans. In this way, the outbreak of the new coronavirus can rest on the Chinese. The viruses in the Middle East are MV2 and H56. No such virus has been detected in China. The seriously ill Iranians said that they had never been to China and had no Chinese friends, but had been to the United States. The world knows that many wealthy people in Middle Eastern countries buy properties in the United States, and their children are also studying in the United States. I am going to write this article today and spread it to the seven major media in the world. My title will read "Americans are the tumors of the world's viruses." The Washington Post published the title of the article last month, stating "Chinese are sick men of East Asia", the birthplace of the virus. I let the world see the hypocrisy, viciousness, loss of conscience, and the spread of the virus to people all over the world. A publication by two Chinese university academics discussed both the WHCDC and the WIV and concluded that “the killer coronavirus probably originated from a laboratory in Wuhan”; the publication was removed from the internet by Chinese government officials. The paper had been posted on Research Gate but was blocked after 24 hours. After being placed on an archive file by internet users, it was again blocked after a week, and the two Chinese authors were pressured to retract the paper. However, it is still available on Web archives.
The 2019-nCoV coronavirus has caused an epidemic of 28,060 laboratory-confirmed infections in human including 564 deaths in China by February 6, 2020. Two descriptions of the virus published on Nature this week indicated that the genome sequences from patients were 96% or 89% identical to the Bat CoV ZC45 coronavirus originally found in
Rhinolophus affinis. It was critical to study where the pathogen came from and how it passed onto human.
May 10, 2018 The top White House official responsible for leading the U.S. response in the event of a deadly pandemic has left the administration, and the global health security team he oversaw has been disbanded under a reorganization by national security adviser John Bolton. The abrupt departure of Rear Adm. Timothy Ziemer from the National Security Council means no senior administration official is now focused solely on global health security. Ziemer’s departure, along with the breakup of his team, comes at a time when many experts say the country is already underprepared for the increasing risks of a pandemic or bioterrorism attack.
“Sadly, we’re not much further than we were weeks and weeks ago,” Pompeo said when asked how the investigation is going. “The Chinese Communist Party has relentlessly deceived and denied information to the west. We have repeatedly asked to have teams go in and assist them to identify where the virus originated. We know it began in Wuhan, but we don’t know from where or from whom, and those are important things. Your listeners need to know. We still need to know that so we can break the code on this thing. One of the key facts for scientists and epidemiologists to build out vaccines and therapeutics and to identify how this was ultimately delivered to the world. You have to know where patient ZERO began and how patient ZERO became infected. Yet, the Chinese Communist Party has at every turn attempted to manage the World Health Organization to manage the information flow, to punish doctors who wanted to talk about this publicly, to undermine the central understandings of transparency that every country has a responsibility to deliver. As a result of that, we still have so many unanswered questions. We, the United States, the world, have so many unanswered questions that are incredibly important so we can address these issues going forward to keep the American people safe. This isn’t political. This isn’t aimed at achieving a political victory. It’s aimed squarely at getting the right health outcomes and saving lives across the world.” Mike Pompeo made the allegations at a private meeting of MPs in London that Dr Tedros Adhanom Ghebreyesus, the organisation’s director-general, had struck a deal with China that helped him secure election.
Mr Pompeo said that “when push came to shove, when it really mattered most”, people had died “because of the deal that was made.” He added that the WHO was a “political” rather than “science-based organisation” that had failed to deal with the Covid-19 pandemic.
His remarks also come in the wake of an announcement by Donald Trump that America would be withdrawing from the WHO, accusing the organisation of being under China’s control. "In early January, for example, China ordered samples of the virus to be destroyed, depriving the world of critical information. Even now, China continues to undermine the International Health Regulations by refusing to share accurate and timely data, viral samples and isolates, and by withholding vital information about the virus and its origins. And, to this day, China
continues to deny international access to their scientists and relevant facilities, all while casting blame widely and recklessly and censoring its own experts...The World Health Organization has failed to publicly call on China to allow for an independent investigation into the origins of the virus, despite the recent endorsement for doing so by its own Emergency Committee. The World Health Organization's failure to do so has prompted World Health Organization member states to adopt the "COVID-19 Response" Resolution at this year's World Health Assembly, which echoes the call by the
United States and so many others for an impartial, independent, and comprehensive review of how the World Health Organization handled the crisis. The resolution also
calls for an investigation into the origins of the virus, which is necessary for the world to understand how best to counter the disease." The spray attacks the virus directly. It contains a lipopeptide, a cholesterol particle linked to a chain of amino acids, the building blocks of proteins. This particular lipopeptide exactly matches a stretch of amino acids in the spike protein of the virus, which the pathogen uses to attach to a human airway or lung cell. NYT The Intelligence Community doesn't rule out a lab leak.
#TedrosOut #WuhanVirus #WuhanCoronavius pic.twitter.com/itvaIOfAm5 "The World Health Organization has expressed concern that US attempts to block publication of aspects of recent research on the H5N1 avian influenza virus may undermine an international agreement on sharing information on the virus. The research relates to genetic mutations that might increase the virus’s risk of transmission to humans. WHO raised its concerns in a statement released on December 30, 2011 and urged that information gathered through such research be shared in the manner delineated in its pandemic influenza preparedness (PIP) framework which came into effect in May 2011." This should not be taken as a sign that @WHO does not think the situation is serious. WHO is following this new #coronavirus outbreak every minute of every day, at country, regional and global level. I will not hesitate to reconvene the committee at a moment’s notice if needed. I thank the Government of #China for its cooperation and transparency. The government has been successful in isolating and sequencing the virus very quickly, and has shared that genetic sequence with @WHO and the international community. #coronavirus Tedros Adhanom Ghebreyesus is an Ethiopian microbiologist and internationally recognized malaria researcher, who has served since 2017 as Director-General of the World Health Organization. Tedros is the first non-physician and first African in the role, who has been endorsed by the African Union. Born: March 3, 1965 (age 55 years), Asmara, Eritrea Nationality: Ethiopian Party: Tigray People's Liberation Front
Tigray Peoples Liberation Front (TPLF) SUMMARY: The Tigrayan People's Liberation Front (TPLF) is a political party in Tigray, Ethiopia that has been listed as a perpetrator in the Global Terrorism Database, based on ten incidents occurring between 1976 and 1990 (see GTD link). Tigray Peoples Liberation Front (TPLF), also known as Popular revolution for the freedom of Tigray, Woyane, Weyane is an active group formed c. 1975. TRAC ANALYSIS: IDEOLOGY Separatist / New Regime Nationalist / Ethnic Nationalist, Left Wing Terrorist Groups (Maoist, Marxist, Communist, Socialist)
TRAC ANALYSIS: TACTICS Attacks on Soft Targets, Assassinations as a Terrorist Tactic TRAC
"Much of the blame for WHO’s failures lies with Dr. Tedros, who is a politician, not a medical doctor. As a member of the left-wing Tigray People’s Liberation Front, he rose through Ethiopia’s autocratic government as health and foreign minister. After taking the director-general job in 2017, he tried to install Zimbabwe dictator Robert Mugabe as a WHO goodwill ambassador. China inevitably gains more international clout as its economy grows. But why does WHO seem so much more afraid of Beijing’s ire than Washington’s? Only 12% of WHO’s assessed member-state contributions come from China. The U.S. contributes 22%. Americans at WHO generally are loyal to the institution, while Chinese appointees put Chinese interests first or they will suffer Beijing’s wrath. To avoid stigmatization against nation and race, the World Health Organization discourages the use of regions, countries, individuals, and animals on naming human infectious diseases and pathogens in its guidelines issued in 2015. The guidelines emphasize that viruses infect all people. That is to say, when an outbreak happens, everyone is at risk, regardless of his or her identity or location. The WHO on February 11 announced the name of the novel coronavirus as SARS-CoV-2 on social media to “prevent the use of other names that can be inaccurate or stigmatizing,” WHO Director-General Tedros Adhanom Ghebreyesus said." "Pneumonia of unknown cause China Disease outbreak news 5 January 2020. On 31 December 2019, the WHO China Country Office was informed of cases of pneumonia of unknown etiology (unknown cause) detected in Wuhan City, Hubei Province of China.According to the authorities, some patients were operating dealers or vendors in the Huanan Seafood market. Based on the preliminary information from the Chinese investigation team, no evidence of significant human-to-human transmission and no health care worker infections have been reported. Based on information provided by national authorities, WHO’s recommendations on public health measures and surveillance of influenza and severe acute respiratory infections still apply.
WHO does not recommend any specific measures for travellers. In case of symptoms suggestive of respiratory illness either during or after travel, travellers are encouraged to seek medical attention and share travel history with their healthcare provider. WHO advises against the application of any travel or trade restrictions on China based on the current information available on this event." "Dr Gauden Galea Director of the Division of Noncommunicable Diseases and Promoting Health through the Life-course Mission. The Division aims to improve health in the WHO European Region by addressing the determinants, risks and consequences of chronic, noncommunicable conditions, by promoting mental and physical wellbeing across the life course, by the population-based prevention and clinical control of disease, and by preventing violence and injury. Biography: Dr Gauden Galea is a public health physician who has worked for WHO since 1998. He has held posts as regional adviser on noncommunicable diseases in the Western Pacific Region, and as coordinator of health promotion in WHO headquarters. Dr Galea has been Director of the Division of Noncommunicable Diseases and Health Promotion at WHO/Europe since January 2011. He has a special interest in health promotion, in the social determinants of noncommunicable diseases, and in the links between these diseases and the development agenda." "The Chinese government has continually violated its promises to us and so many other nations these plain facts cannot be overlooked or swept aside. The world is now suffering as a result of the
malfeasance of the Chinese government. China's cover-up of the Wuhan virus allowed the disease to spread all over the world instigating a global pandemic that has cost more than 100,000 in American lives and over a million lives worldwide. Chinese officials ignored their reporting obligations to the World Health Organization and pressured the World Health Organization to mislead the world when the virus was first discovered by Chinese authorities. Countless lives have been taken and profound economic hardship has been inflicted all around the globe. They strongly recommended against me doing
the early ban from China but I did it anyway it was proven to be 100% correct. China has total control over the World Health Organization despite only paying 40 million dollars per year compared to what the United States has been paying which is approximately 450 million dollars a year. We have detailed the reforms that it must make and engage with them directly but they have refused to act because they have failed to make the requested and greatly needed reforms. We will be today terminating our relationship with the World Health Organization and redirecting those funds to other worldwide and serving urgent global public health needs. The world needs answers from China on the virus. We must have transparency. Why is it that China shut off infected people from Wuhan to all other parts of China. It went nowhere else. It didn't go to Beijing it went nowhere else but they allowed them to freely travel throughout the world including Europe and the United States. The death and destruction caused by this is incalculable. We must have answers not only for us but for the rest of the world. This pandemic has underscored the crucial importance of building up America's economic independence reshoring our critical supply chains and protecting America's scientific and technological advances. For years the government of China has conducted illicit espionage to steal our industrial secrets of which there are many today I will issue a proclamation to better secure our nation's vital university research and to suspend the entry of certain foreign nationals from China who we have identified as potential security risks." The World Health Organisation announced it would send a team into China to investigate the source of the pathogen, which has now killed more than half a million people. “We can fight the virus better when we know everything about the virus, including how it started,” WHO director general Tedros Adhanom Ghebreyesus said in Geneva. “We will be sending a team next week to China to prepare for that.” The level of access afforded the WHO team in China could depend on who was on the team and whether Beijing perceived it as neutral, Yanzhong Huang, a senior fellow for global health at the Council on Foreign Relations in New York, said. He also noted that China would not want to be perceived as uncooperative. In his comments on Monday, Tedros gave little detail about the mission or whether it would be run as a joint body also led by a Chinese health official, as was the case for the WHO team visit in February. No matter when an independent forensic investigation gets underway (if that ever happens), the questions of how the pandemic began and why it wasn’t quashed in infancy may take years to answer. Still, it’s already clear that the developed world—with all of its advanced medical and communications technologies and national and international governance structures—undeniably and miserably failed to stop a lethal disease from infecting the globe and exacting enormous human and financial costs.
The virus lingers in the air indoors, infecting those nearby. If airborne transmission is a significant factor in the pandemic, especially in crowded spaces with poor ventilation, the consequences for containment will be significant. Masks may be needed indoors, even in socially-distant settings. Health care workers may need N95 masks that filter out even the smallest respiratory droplets as they care for coronavirus patients. The World Health Organization has long held that the coronavirus is spread primarily by large respiratory droplets that, once expelled by infected people in coughs and sneezes, fall quickly to the floor. Even in its latest update on the coronavirus, released June 29, 2020 the W.H.O. said airborne transmission of the virus is possible only after medical procedures that produce aerosols, or droplets smaller than 5 microns. (A micron is equal to one millionth of a meter.) Proper ventilation and N95 masks are of concern only in those circumstances, according to the W.H.O. Instead, its infection control guidance, before and during this pandemic, has heavily promoted the importance of handwashing as a primary prevention strategy, even though there is limited evidence for transmission of the virus from surfaces. (The Centers for Disease Control and Prevention now says surfaces are likely to play only a minor role.) Dr. Benedetta Allegranzi, the W.H.O.’s technical lead on infection control, said the evidence for the virus spreading by air was unconvincing. “Especially in the last couple of months, we have been stating several times that we consider airborne transmission as possible but certainly not supported by solid or even clear evidence,” she said. “There is a strong debate on this.” But interviews with nearly 20 scientists — including a dozen W.H.O. consultants and several members of the committee that crafted the guidance — and internal emails paint a picture of an organization that, despite good intentions, is out of step with science.
NY TIMES
Kristian Andersen, PhD, a professor in the Department of Immunology and Microbiology at Scripps Research Institute in La Jolla, California, and lead author of a research letter published Mar 17 in Nature Medicine on the origins of the virus, first thought that SARS-CoV-2 was just as likely to have been accidentally released from a lab as it was to have come from nature. But he changed his mind and based on their genomic sequencing analysis, he concluded that the most likely origins for SARS-CoV-2 followed one of two possible scenarios.
"In one scenario, the virus evolved to its current pathogenic state through natural selection in a non-human host and then jumped to humans. This is how previous coronavirus outbreaks have emerged, with humans contracting the virus after direct exposure to civets (SARS) and camels (MERS). The researchers proposed bats as the most likely reservoir for SARS-CoV-2 as it is very similar to a bat coronavirus. There are no documented cases of direct bat-human transmission, however, suggesting that an intermediate host was likely involved between bats and humans. In this scenario, both of the distinctive features of SARS-CoV-2's spike protein -- the receptor-binding domain portion that binds to cells and the cleavage site that opens the virus up -- would have evolved to their current state prior to entering humans. In this case, the current epidemic would probably have emerged rapidly as soon as humans were infected, as the virus would have already evolved the features that make it pathogenic and able to spread between people." That a mighty quick evolution between SARS-CoV-1 and SARS-CoV-2. Operation Warp Speed?
"In the other proposed scenario, a non-pathogenic version of the virus jumped from an animal host into humans and then evolved to its current pathogenic state within the human population. For instance, some coronaviruses from pangolins, armadillo-like mammals found in Asia and Africa, have a receptor-binding domain structure very similar to that of SARS-CoV-2. A coronavirus from a pangolin could possibly have been transmitted to a human, either directly or through an intermediary host such as civets or ferrets. Then the other distinct spike protein characteristic of SARS-CoV-2, the cleavage site, could have evolved within a human host, possibly via limited undetected circulation in the human population prior to the beginning of the epidemic. The researchers found that the SARS-CoV-2 cleavage site, appears similar to the cleavage sites of strains of bird flu that has been shown to transmit easily between people. SARS-CoV-2 could have evolved such a virulent cleavage site in human cells and soon kicked off the current epidemic, as the coronavirus would possibly have become far more capable of spreading between people."
The virus evolved inside its human host? 8% of the human genome consists of retrovirus fragments so this is theoretically possible. But patient ZERO was a man advanced in years. One would assume it made this jump in someone who worked in the Wuhan Wet Market and lot of contact with wildlife. But the market has been eliminated as the birthplace of the virus so so "Hans" Kristain Anderson's theory is another fairy tale. Scientists have published the genetic sequence of virus taken from about 100 patients. They show mutations taking place at a slow pace as the infection passes from person to person. Typically the virus in one patient today is different in around five of the 30,000 biochemical letters of its genetic code, but these are random changes rather than any sign that it is becoming more virulent or infectious, cancer researcher Dr. Trevor Bedford said. SARS-CoV-1 started appearing in 2002. The virus need about 50 years to mutate to SARS-CoV-2.
The variant discovered in Britain, known as B.1.1.7, has 23 mutations that differ from the earliest known version of the virus in Wuhan, China, including one or more that make it more contagious, and at least one that slightly weakens the vaccines’ potency. Some experiments suggest that the variant spreads more easily because mutations enable it to latch more successfully onto a person’s airway. One mutation the patient had, labeled 69-70del, changes the shape of the spike protein. Another, N501Y, can help the protein bind more tightly to human cells. Eventually, British scientists detected 23 mutations that distinguished these genomes from the earliest known version in Wuhan, China — enough to be a considered a new variant, since labeled B.1.1.7. On an evolutionary tree that Dr. Kemp made, it stood apart like a lone, spindly branch.NYT
Our analyses clearly show that SARS-CoV-2 is not a laboratory construct or a purposefully manipulated virus. SARS-CoV-2 appears to be optimized for binding to the human receptor ACE2 and (ii) the spike protein of SARS-CoV-2 has a functional polybasic (furin) cleavage site PRRA at the S1–S2 boundary through the insertion of 12 nucleotides, which additionally led to the predicted acquisition of three O-linked glycans around the site. On the basis of structural studies7,8,9 and biochemical experiments1,9,10, SARS-CoV-2 seems to have an receptor-binding domain that binds with high affinity to ACE2 from humans, ferrets, cats and other species with high receptor homology. While the analyses above suggest that SARS-CoV-2 may bind human ACE2 with high affinity, computational analyses predict that the interaction is not ideal and that the receptor-binding domain sequence is different from those shown in SARS-CoV to be optimal for receptor binding. Thus, the high-affinity binding of the SARS-CoV-2 spike protein to human ACE2 is most likely the result of natural selection on a human or human-like ACE2 that permits another optimal binding solution to arise. This is strong evidence that SARS-CoV-2 is not the product of purposeful manipulation.
Polybasic cleavage sites have not been observed in related ‘lineage B’ betacoronaviruses, although other human betacoronaviruses, including HKU1 (lineage A), have those sites and predicted O-linked glycans. Given the level of genetic variation in the spike, it is likely that SARS-CoV-2-like viruses with partial or full polybasic cleavage sites will be discovered in other species.
The functional consequence of the polybasic cleavage site PRRA in SARS-CoV-2 is unknown, and it will be important to determine its impact on transmissibility and pathogenesis in animal models. Experiments with SARS-CoV have shown that insertion of a furin cleavage site at the S1–S2 junction enhances cell–cell fusion without affecting viral entry1
It is improbable that SARS-CoV-2 emerged through laboratory manipulation of a related SARS-CoV-like coronavirus. As noted above, the receptor-binding domain of SARS-CoV-2 is optimized for binding to human ACE2 with an efficient solution different from those previously predicted. Furthermore, if genetic manipulation had been performed, one of the several reverse-genetic systems available for betacoronaviruses would probably have been used. However, the genetic data irrefutably show that SARS-CoV-2 is not derived from any previously used virus backbone.
Genetic analysis shows the virus began to spread sometime in the fall or winter of 2019, says Robert Garry, a microbiologist at Tulane University. Those same analyses refuted an earlier theory that the virus was genetically engineered in a laboratory. Garry says the reason is simple — the virus infects people in a way that scientists had never seen before: "The virus is just really too good at what it's doing," he says. "No human using a computer could do this. This is very clearly a natural process that occurred." In addition, he says, there are no signs of human genetic modification in the virus's code. Angela Rasmussen, PhD, an associate research scientist in the Center for Infection and Immunity at Columbia University in New York City, said computer modeling suggests that the receptor-binding domain of the spike protein in SARS-CoV-2, the virus that causes SARS-CoV-2, is suboptimal, "meaning that someone designing an optimal receptor-binding domain sequence probably would not 'engineer' the sequence that evolved in SARS-CoV-2," she said. Today in the US we will have: The GeneArt Seamless PLUS Cloning and Assembly Technology is a highly efficient, vector-independent system for the simultaneous and seamless assembly of up to 4 DNA inserts between 100 bp and 10 kb and any vector, totaling up to 40 kb in length. The system allows the cloning of the DNA inserts into virtually any linearized E. coli vector, requires no pre-existing recombination sites or extra DNA sequences, and eliminates the need for extensive enzymatic treatments of the DNA such as restriction and ligation. The enzyme mix provided with the GeneArt Seamless PLUS Cloning and Assembly Kit recognizes and precisely assembles the DNA inserts sharing an end-terminal homology of between 15 bp and 80 bp, depending on the total construct size. The GeneArt Seamless Cloning and Assembly Kit enables the simultaneous and directional cloning of 1 to 4 PCR fragments, consisting of any sequence, into any linearized vector, in a single 30-minute room temperature reaction. The kit contains everything required for the assembly of DNA fragments, and their transformation into E. coli for selection and growth of recombinant vectors They made it come back? https://t.co/uHhwucnMD9 director@covid19researchinstitute.com THE STATE DEPARTMENT CABLES: THE TECHNICIANS AT WIV ARE DUMMIES
LACK OF CELL PHONE ACTIVITY AT WIV
A report — obtained by the London-based NBC News Verification Unit — says there was no cellphone activity in a high-security portion of the Wuhan Institute of Virology from Oct. 7 through Oct. 24, 2019, and that there may have been a "hazardous event" sometime between Oct. 6 2019 and Oct. 11 2019. "Would be interesting if someone analyzed commercial telemetry data at & near Wuhan lab from Oct-Dec 2019," Marco Rubio tweeted. "If it shows dramatic drop off in activity compared to previous 18 months it would be a strong indication of an incident at lab & of when it happened."
WIV WAS CALLING IN OUTSIDERS TO HELP WITH BIOSAFETY

THE BATLADY CREATED COVID-19 IN A LEVEL 2 BIOCONTAINMENT LAB, AND IT LEAKED OR AN INFECTED PERSON, THE UKNOWN PATIENT ZERO SYMPTOMATIC OR NON-SYMPTOMATIC WHO WORKED AT THE WIV AND TRANSMITTED IT INTO WUHAN
PETER DASZAK who ran the CIA / USAID Operation EcoHealth financed WIV research, SHI ZHENG LI created the virus and WANG YANYI and WEIFENG SHI covered it up. These scientists were inducing GoF mutations, doing genetic insertions and assembling new parts for the SARS CoV-1 virus and thus creating new forms of life at the time the first case of SARS-CoV-2 appeared. It was SHI ZHENG LI's team that produced SARS-CoV-2.

SHI ZHENG LI WORK AT THE TIME OF THE OUTBREAK INVOLVED STUDYING CORONA VIRUSES ALMOST IDENTICAL TO SARS Co-V-2
SHI ZHENG LI CHRONOLOGY


2018 SHI ZHENG LI SYNTHETIC VIRUSES
https://www.med.unc.edu/orfeome/files/2018/03/a-sars-like-cluster-of-circulating-bat-coronaviruses-shows-potential-for-human-emergence.pdf
SHI ZHENG LI POST SARS-CoV-2 IN SCIENCE MAGAZINE
SHING HEI ZHAN, BENJAMIN E. DEVERMAN AND YUJIA ALINA CHAN, “SARS-COV-2 IS WELL ADAPTED FOR HUMANS
THE INTERVIEW WITH THE BAT LADY WHO SAYS COVID IS PUNISHMENT FOR AN UNSANITARY LIFESTYLE
DESTRUCTION OF SARS-COV-2 SAMPLES

Note: Science asked two follow-up questions after receiving the replies above.
DEFECTION
"RECTIFICATION" OF LAB THAT MAPPED GENOME
NEW YORK TIMES 9/20/2020 CHINA VIRUS STILL NOT FOUND IN NATURE

THE PROXIMITY FACTOR
PATIENT ZERO

THE FORENSIC EVIDENCE OF LAB CREATION
GAIN OF FUNCTION INSERTIONS AT POLYBASIC FURIN CLEAVAGE SITE IS EVIDENCE VIRUS WAS MODIFIED FOR INCREASED INFECTIVITY AND PATHOGENICITY
DR. RONEN SHEMESH EXPLAINS THE SIGNIFICANCE OF THE POLYBASIC FURIN CLEAVAGE SITE PRRA

UNIQUE NEW FEATURE OF SARS-CoV-2 CALLED "UNEXPECTED INSERTION"
HUMAN ENGINEERING OF A POLYBASIC HA CLEAVAGE SITE MUTANT WAS EASILY ACCOMPLISHED
BtCoV/4991, RaTG13, RmYN02 THE MYSTERIOUS BAT VIRUSES SUPPOSEDLY SIMILAR TO SARS-CoV-2

WEIFENG SHI'S RmYN02 VIRUS THAT HAS DISFUNCTIONAL APPROXIMATION TO POLYBASIC CLEAVAGE SITE PRRA TO PROVE SARS-CoV-2 THIS MUTATION IS FOUND IN NATURE NOT JUST IN THE LAB
SHI ZHENG LI CALLS THE VIRUSES SIMILAR DESPITE SIGNIFICANT DIFFERENCE: SARS-CoV-2 IS INFECTIOUS RmYN02 IS NOT
SHI ZHENG LI COMMENTS
SHI ZHENG LI RaTG13 ALLEGEDLY FOUND IN NATURE, UNAVAILABLE TO THE SCIENTIFIC COMMUNITY
* Single Nucleotide Polymorphisms (SNPs) are the most common type of genetic variation among people. Each SNP represents a difference in a single DNA building block, called a nucleotide.
THE CHINESE FAILED TO REVEAL THE EXISTENCE OF RaTG13 UNTIL 2020 AND RaTG13 IS ANOTHER NAME FOR BtCoV/4991
MAY 24, 2020 SOUTH CHINA MORNING POST RESEARCHERS IDENTIFIED THE GENOME SEQUENCE OF RaTG-13 THEY DID NOT ISOLATE NOR OBTAIN THE LIVE VIRUS OF RATG-13.
SARS-CoV-2 WAS NEVER FOUND IN THE BLOOD OR FECES OF ANY ANIMAL
SARS-CoV-1 WAS FOUND IN ANIMALS
SHI ZHENG LI 2015 EXPERIMENTS SYNTHESIZING VIRUSES THAT ATTACK ACE2RECEPTORS IN HUMANS WERE PRECURSORS TO CREATION OF SARS-CoV-2

*A titer is a ratio used to explain the amount of something in a solution. When talking about vaccines or immunity to disease, titers identify the amount of antibodies in a person’s blood.
2015: ISOLATION AND CHARACTERIZATION OF A NOVEL BAT CORONA VIRUS CLOSELY RELATED TO THE DIRECT PROGENITOR OF SARS: CREATION OF A VIRUS THAT CAN USE ACE2AS RECEPTOR
SHI ZHENG LI 2015 EXPERIMENTS IN UNIVERSITY NORTH CAROLINA, CHAPEL HILL LAB PRODUCED NOVEL VIRUS MORE DEADLY TO OLDER PEOPLE JUST LIKE SARS-CoV-2 AND STIR DEBATE ABOUT GoF RESEARCH CAUSING A PANDEMIC 2020: NATIONAL INSTITUTE OF HEALTH SL-SHC014-MA15: MAN MADE VIRUS WHICH USES ACE2RECEPTOR
Due to the elevated pathogenic activity of the SL-SHC014-MA15 chimeric virus relative to MA15 chimeric virus with the original human SARS S gene in mice, such experiments with SL-SHC014-MA15 chimeric virus were later restricted as gain of function (GOF) studies under the US government-mandated pause policy.
The current SARS-CoV-2 epidemic has restarted the debate over the risks of constructing such viruses that could have pandemic potential, irrespective of the finding that these bat CoVs already exist in nature. Regardless, upon careful phylogenetic analyses by multiple international groups the SARS-CoV-2 is undoubtedly distinct from SL-SHC014-MA15 with >6,000 nucleotide differences across the whole genome. Therefore, once again there is no credible evidence to support the claim that the SARS-CoV-2 is derived from the chimeric SL-SHC014-MA15 virus. COMMENT: It could have used as a backbone if RaTG13 didn't exist. DECEMBER 2015: SHI ZHENG LI EXPERIMENTS ARE POTENTIAL PANDEMIC VECTOR: "SARS-LIKE CLUSTER OF CIRCULATING BAT CORONAVIRUS POSE THREAT FOR HUMAN EMERGENCE" XING-YI GE, ZHENGLI-LI SHI, AND RALPH S. BARIC

NATURE: SL-SHC014-MA15 STIRS DEBATE OVER RISKY RESEARCH: LAB-MADE CHIMAERA SIMILAR TO SARS-CoV-2 CAN INFECT ACE2CELLS

UNIVERSITY OF NORTH CAROLINA PRESS RELEASE

WHY THIS VIRUS IS DIFFERENT THAN ALL OTHER CORONA VIRUSES?
SARS-CoV-1 IS GREASY KIDS STUFF COMPARED TO SARS-CoV-2
SYMPTOMS OF SARS-1 DID NOT ENTAIL CAUSING EVERY DISEASE IN A MEDICAL ENCYLOPEDIA
GoF MADE A SARS-CoV-1 MORE TRANSMITTABLE AND MORE LETHAL
This is my 5 yrs old great niece. She was fit & healthy until a mild bout of SARS-CoV-2 5wks ago from which she appeared to recover.
She is now in ICU with a Kawasaki inflammatory response.
She is off the ventilator but has developed heart problems.1/#Marr #Ridge#COVID19 pic.twitter.com/AG9b7k4Sgn NEW STUDY HAS WARNED THAT POTENTIALLY DEADLY BRAIN DISORDERS MAY BE A SYMPTOM OF COVID-19, EVEN IN PEOPLE WITH OTHERWISE MILD DISEASE.
says: "Gain of Function or Gain of Purpose research: scientists purposefully cause mutations that give viruses new abilities in an attempt to better understand the pathogen. Flu viruses are basically eight pieces of RNA wrapped up in a ball. To create the gain-of-function mutations, one uses a DNA template for each piece, called a plasmid. Making a single mutation in the plasmid is easy and it's commonly done in genetics labs. If you insert all eight plasmids into a mammalian cell, they hijack the cell's machinery to create flu virus RNA. Now you can start to assemble a new virus particle in that cell,"One infected cell is enough to grow many new virus particles — from one to a thousand to a million; viruses are replication machines. And because they mutate so readily during their replication, the new viruses have to be checked to make sure it only has the mutations the lab caused. The virus then goes into the ferrets, passing through them to generate new viruses until, on the 10th generation, it infected ferrets through the air. By analyzing the virus's genes in each generation, they can figure out what exact five mutations lead to H5N1 bird flu being airborne between ferrets. And, potentially, people.
RON FOUCHIER: MAKES AVIAN FLU VIRUS TRANMITTABLE TO HUMANS. WHAT THE FUCK FOR?

The potential for the modified H5N1 strain to cause a human pandemic if it ever slipped out of containment has sparked sharp criticism and no shortage of controversy. Rutgers molecular biologist Richard Ebright summed up the far end of the opposition when he told Science that the research "should never have been done." "When I first heard about the experiments that make highly pathogenic avian influenza transmissible," says Philip Dormitzer, vice president and chief scientific officer of viral vaccines at Pfizer, "I was interested in the science but concerned about the risks of both the viruses themselves and of the consequences of the reaction to the experiments." In 2014, in response to researchers' fears and some lab incidents, the federal government imposed a moratorium on all GOF research, freezing the work.
2011 ANTHONY FAUCI HELPED ENGINEER SARS-CoV-2 BY OPPOSING GoF BAN
"Important information and insights can come from generating a potentially dangerous virus in the laboratory."
WASHINGTON POST EDITORIAL 2011
ANTHONY S. FAUCI'S EXPERIMENTS INVOLVED DURC: DUAL USE RESEARCH OF CONCERN
TEXAS COLLEGES AND UNIVERSITIES IN BED WITH WIV THANKS TO FAUCI GRANT
National Science Advisory Board for Biosecurity (NSABB)
James W. Le Duc, PhD
Director, Galveston National Laboratory
Professor, Department of Microbiology and
Immunology
University of Texas Medical Branch
OBAMA BANS GAIN-OF-FUNCTION RESEARCH IN US OCTOBER 2014
ONE OF SEVERAL NEAR FATAL ERRORS THAT TRIGGERED THE GOF BAN INVOLVED H5N1 INFLUENZA VIRUS
THE SARS ESCAPES
BULLETIN OF ATOMIC SCIENTISTS: PROBABILITY OF ACCIDENTAL RELEASE INTO THE COMMUNITY
GAIN-OF-FUNCTION OUTSOURCED TO WUHAN INSTITUTE OF VIROLOGY 2014-2019 AFTER AMERICA BANS IT AS TOO DANGEROUS
https://www.snopes.com/fact-check/obama-admin-wuhan-lab-grant
TRUMP LIFTS GAIN-OF-FUNCTION BAN LATE 2017
"On Dec 19, 2017, the US National Institutes of Health (NIH) announced that they would resume funding gain-of-function experiments involving influenza, Middle East respiratory syndrome coronavirus, and severe acute respiratory syndrome coronavirus. A moratorium had been in place since October, 2014. At the time, the NIH had stated that the moratorium “will be effective until a robust and broad deliberative process is completed that results in the adoption of a new US Government gain-of-function research policy.” This process has now concluded. It was spearheaded by the National Science Advisory Board for Biosecurity (NSABB) and led to the development of a new framework for assessing funding decisions for research involving pathogens with enhanced pandemic potential. The release of the framework by the Department of Health and Human Services (HHS), of which NIH is part, signalled the end of the funding pause." Francis Collins, M.D., Ph.D. Director, National Institutes of Health."
THE WET MARKET MYTH: VIRUS WAS NEVER FOUND IN ANIMALS AT THE WET MARKET OR ANYWHERE ELSE

HARVARD UNIVERSITY STUDY
NEW YORKER MAGAZINE


THE PANGOLIN SCAPEGOAT
SARS-CoV-2 WAS NEVER FOUND IN PANGOLINS

THE BATS WERE IN THEIR WINTER HIBERNATION PERIOD WHEN SARS-CoV-2 FIRST APPEARED
THE LANCET NEW ENGLAND JOURNAL OF MEDICINE
SCIENTIFIC AMERICAN

USAID Shuttered Disease Surveillance Program October 28, 2019. "The federal government has shut down USAID’s PREDICT surveillance program that sought out threatening animal viruses that could leap to human populations. In its 10 years of existence, PREDICT cost $207 million and collected 140,000 samples from animals and discovered more than 1000 viruses. Another achievement: It trained 5000 people in 30 countries in Africa and Asia. PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing. That’s expensive,” said PETER DASZAK, the EcoHealth Alliance’s president. “The United States spent $5 billion fighting Ebola in West Africa.”

WANG YANYI RULES OUT ANIMAL MARKET AND LAB AS CORONAVIRUS ORIGIN

CHINESE VERIFY THEIR INTEREST IN DASZAK WHO FINANCED EXPERIMENTS THAT HELPED CREATE SARS-CoV-2

PETER DASZAK LACKS DEGREE IN MEDICINE
DASZAK: "LOOK, FIRST, THE IDEA THAT THIS VIRUS ESCAPED FROM A LAB IS JUST PURE BALONEY. IT’S SIMPLY NOT TRUE. I’VE BEEN WORKING WITH THAT LAB FOR 15 YEARS.
CHINA DAILY
CHI COM PROPAGANDA ORGAN QUOTES PETER DASZAK
DASZAK INTERVIEW: THEN THERE ARE HEROS LIKE SHI ZHENG LI IN CHINA, WHO HAS STUCK THROUGH DEATH THREATS AND A COMPLETE DISPARAGING OF HER CHARACTER, JUST TO DO WHAT SHE DOES: TRY TO SAVE LIVES


TRUMP CUTS OFF FUNDS TO ECOHEALTH ALLIANCE 2020
SOME OF USAID/CIA ECO-HEALTH CREW


DASZAK TWEETS
CHICOM RE-ACTION DISINFORMATION CAMPAIGN SUPPRESSION OF INFORMATION AND INDIVIDUALS BY CHINESE AUTHORITIES.
An article published on The Lancet reported that 41 people in Wuhan were found to have the acute respiratory syndrome and 27 of them had contact with Huanan Seafood Market 3. The 2019-nCoV was found in 33 out of 585 samples collected in the market after the outbreak. The market was suspicious to be the origin of the epidemic, and was shut down according to the rule of quarantine the source during an epidemic.
The bats carrying CoV ZC45 were originally found in Yunnan or Zhejiang province, both of which were more than 900 kilometers away from the seafood market. Bats were normally found to live in caves and trees. But the seafood market is in a densely-populated district of Wuhan, a metropolitan of ~15 million people. The probability was very low for the bats to fly to the market. According to municipal reports and the testimonies of 31 residents and 28 visitors, the bat was never a food source in the city, and no bat was traded in the market. There was possible natural recombination or intermediate host of the coronavirus, yet little proof has been reported.
Was there any other possible pathway? We screened the area around the seafood market and identified two laboratories conducting research on bat coronavirus. Within ~280 meters from the market, there was the Wuhan Center for Disease Control & Prevention (WHCDC) WHCDC hosted animals in laboratories for research purpose, one of which was specialized in pathogens collection and identification. In one of their studies, 155 bats including Rhinolophus affinis were captured in Hubei province, and other 450 bats were captured in Zhejiang province 4. The expert in collection
was noted in the Author Contributions (JHT). Moreover, he was broadcasted for collecting viruses on nation-wide newspapers and websites in 2017 and 2019. He described that
he was once by attacked by bats and the blood of a bat shot on his skin. He knew the extreme danger of the infection so he quarantined himself for 14 days. In another accident,
he quarantined himself again because bats peed on him. He was once thrilled for capturing a bat carrying a live tick. Surgery was performed on the caged animals and the tissue samples were collected for
DNA and RNA extraction and sequencing. The tissue samples and contaminated trashes were source of pathogens. They were only ~280 meters from the seafood market. The WHCDC was also adjacent to the Union Hospital ) where the first group of doctors were infected during this epidemic. It is plausible that the virus leaked around and some of them contaminated the initial patients in this epidemic, though solid proofs are needed in future study.
The second laboratory was ~12 kilometers from the seafood market and belonged to Wuhan Institute of Virology, Chinese Academy of Sciences. This laboratory reported that the Chinese horseshoe bats were natural reservoirs for the severe acute respiratory syndrome coronavirus (SARS-CoV) which caused the 2002-3 pandemic. The principle investigator participated in a project which generated a chimeric virus using the SARS-CoV reverse genetics system, and reported the potential for human emergence 10. A direct speculation was that SARS-CoV or its derivative might leak from the laboratory.
In summary, somebody was entangled with the evolution of 2019-nCoV coronavirus. In addition to origins of natural recombination and intermediate host, the killer coronavirus probably originated from a laboratory in Wuhan. Safety level may need to be reinforced in high risk biohazardous laboratories. Regulations may be taken to relocate these laboratories far away from city center and other densely populated places.

BOLTON AND TRUMP PAVE THE WAY FOR THE PANDEMIC
TRUMP WANTED AN INVESTIGATION OF THE ORIGIN OF THE VIRUS
ANOTHER POSSIBILITY WHICH STILL CANNOT BE EXCLUDED IS THAT SARS-CoV-2 WAS CREATED BY A RECOMBINATION EVENT THAT OCCURRED INADVERTENTLY OR CONSCIOUSLY IN A LABORATORY HANDLING CORONAVIRUSES, WITH THE NEW VIRUS THEN ACCIDENTALLY RELEASED INTO THE LOCAL HUMAN POPULATION."

WHO


WORLD HEALTH ORGANIZATION IS IN FAVOR OF SHARING H5N1 VIRUS GAIN-OF-FUNCTION RESEARCH 2012
REFERENCES
http://www.who.int/mediacentre/news/statements/2011/pip_framework_20111229/en/index.html
http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_8-en.pdf
https://www.bmj.com/content/344/bmj.e30.full
https://doi.org/10.1136/bmj.e30 (Published 04 January 2012)
WORLD HEALTH ORGANIZATION CULPABILITY: HEAD OF WHO IS MAOIST CHINESE AGENT OF INFLUENCE
WALL STREET JOURNAL
EARLY WHO REPORT
WHO REPRESENTATIVE TO CHINA DURING EARLY PHASE OF PANDEMIC WAS EXPERT IN NONCOMMUNICABLE DISEASES
TRUMP PULLS USA OUT OF WHO
NEW WHO JUNKET
WHO DISPUTES THE FACT THE VIRUS LINGERS IN THE AIR INDOORS, INFECTING THOSE NEARBY.
KRISTIAN ANDERSON FAIRY TALES ON THE ORIGINS OF THE VIRUS

THE MANY VARIANTS - EUPHEMISM FOR MUTATION - THAT HAVE EMERGED SHOW THAT BEDFORD IS A WRONG
WHILE THE ANALYSES ABOVE SUGGEST THAT SARS-COV-2 MAY BIND HUMAN ACE2WITH HIGH AFFINITY, COMPUTATIONAL ANALYSES PREDICT THAT THE INTERACTION IS NOT IDEAL SO IT COULD NOT BE MAN-MADE OTHERWISE THE BINDING WOULD BE IDEAL?
THE VIRUS WAS NOT MAN MADE BECAUSE IT IS LIKELY THAT SARS-COV-2-LIKE VIRUSES WITH PARTIAL OR FULL POLYBASIC CLEAVAGE SITE PRRA WILL BE DISCOVERED IN OTHER SPECIES.
INSERTION OF A FURIN CLEAVAGE SITE AT THE S1–S2 JUNCTION ENHANCES CELL–CELL FUSION WITHOUT AFFECTING VIRAL ENTRY IS FALSE
SARS-CoV-2 COULD HAVE BEEN DERIVED FROM A NEW VIRUS BACKBONE
NPR: VIRUS IS TOO DEADLY TO BE COMPUTER GENERATED
ANDERSON'S BIAS AGAINST TRUMP AFFECTS HIS RESEARCH
1. The most daily COVID deaths in months
2. The largest drop in GDP ever
3. A president suggesting delaying an election
4. A former presidential candidate dying from COVID
Time for change, maybe?
CCP PROPAGANDA